Activity-independent prespecification of synaptic partners in the visual map of Drosophila
- PMID: 16979562
- PMCID: PMC3351197
- DOI: 10.1016/j.cub.2006.07.047
Activity-independent prespecification of synaptic partners in the visual map of Drosophila
Abstract
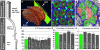
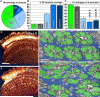
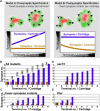
Specifying synaptic partners and regulating synaptic numbers are at least partly activity-dependent processes during visual map formation in all systems investigated to date . In Drosophila, six photoreceptors that view the same point in visual space have to be sorted into synaptic modules called cartridges in order to form a visuotopically correct map . Synapse numbers per photoreceptor terminal and cartridge are both precisely regulated . However, it is unknown whether an activity-dependent mechanism or a genetically encoded developmental program regulates synapse numbers. We performed a large-scale quantitative ultrastructural analysis of photoreceptor synapses in mutants affecting the generation of electrical potentials (norpA, trp;trpl), neurotransmitter release (hdc, syt), vesicle endocytosis (synj), the trafficking of specific guidance molecules during photoreceptor targeting (sec15), a specific guidance receptor required for visual map formation (Dlar), and 57 other novel synaptic mutants affecting 43 genes. Remarkably, in all these mutants, individual photoreceptors form the correct number of synapses per presynaptic terminal independently of cartridge composition. Hence, our data show that each photoreceptor forms a precise and constant number of afferent synapses independently of neuronal activity and partner accuracy. Our data suggest cell-autonomous control of synapse numbers as part of a developmental program of activity-independent steps that lead to a "hard-wired" visual map in the fly brain.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

