Loss of CNGB1 protein leads to olfactory dysfunction and subciliary cyclic nucleotide-gated channel trapping
- PMID: 16980309
- PMCID: PMC2885922
- DOI: 10.1074/jbc.M606409200
Loss of CNGB1 protein leads to olfactory dysfunction and subciliary cyclic nucleotide-gated channel trapping
Abstract
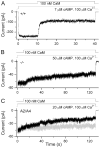
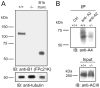
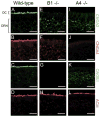
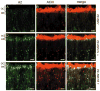
Olfactory receptor neurons (ORNs) employ a cyclic nucleotide-gated (CNG) channel to generate a receptor current in response to an odorant-induced rise in cAMP. This channel contains three types of subunits, the principal CNGA2 subunit and two modulatory subunits (CNGA4 and CNGB1b). Here, we have analyzed the functional relevance of CNGB1 for olfaction by gene targeting in mice. Electro-olfactogram responses of CNGB1-deficient (CNGB1-/-) mice displayed a reduced maximal amplitude and decelerated onset and recovery kinetics compared with wild-type mice. In a behavioral test, CNGB1-/- mice exhibited a profoundly decreased olfactory performance. Electrophysiological recordings revealed that ORNs of CNGB1-/- mice weakly expressed a CNG current with decreased cAMP sensitivity, very rapid flicker-gating behavior and no fast modulation by Ca2+-calmodulin. Co-immunoprecipitation confirmed the presence of a CNGA2/CNGA4 channel in the olfactory epithelium of CNGB1-/- mice. This CNGA2/CNGA4 channel was targeted to the plasma membrane of olfactory knobs, but failed to be trafficked into olfactory cilia. Interestingly, we observed a similar trafficking defect in mice deficient for the CNGA4 subunit. In conclusion, these results demonstrate that CNGB1 has a dual function in vivo. First, it endows the olfactory CNG channel with a variety of biophysical properties tailored to the specific requirements of olfactory transduction. Second, together with the CNGA4 subunit, CNGB1 is needed for ciliary targeting of the olfactory CNG channel.
Figures

Similar articles
-
Calcium/calmodulin modulation of olfactory and rod cyclic nucleotide-gated ion channels.J Biol Chem. 2003 May 23;278(21):18705-8. doi: 10.1074/jbc.R300001200. Epub 2003 Mar 7. J Biol Chem. 2003. PMID: 12626507 Review.
-
Simultaneous Loss of NCKX4 and CNG Channel Desensitization Impairs Olfactory Sensitivity.J Neurosci. 2017 Jan 4;37(1):110-119. doi: 10.1523/JNEUROSCI.2527-16.2016. J Neurosci. 2017. PMID: 28053034 Free PMC article.
-
Deciphering the function of the CNGB1b subunit in olfactory CNG channels.Sci Rep. 2016 Jul 11;6:29378. doi: 10.1038/srep29378. Sci Rep. 2016. PMID: 27405959 Free PMC article.
-
Differential regulation by cyclic nucleotides of the CNGA4 and CNGB1b subunits in olfactory cyclic nucleotide-gated channels.Sci Signal. 2012 Jul 10;5(232):ra48. doi: 10.1126/scisignal.2003110. Sci Signal. 2012. PMID: 22786723
-
Cyclic nucleotide-gated ion channels.Physiol Rev. 2002 Jul;82(3):769-824. doi: 10.1152/physrev.00008.2002. Physiol Rev. 2002. PMID: 12087135 Review.
Cited by
-
Inherited macular degeneration-associated mutations in CNGB3 increase the ligand sensitivity and spontaneous open probability of cone cyclic nucleotide-gated channels.Front Physiol. 2015 Jun 9;6:177. doi: 10.3389/fphys.2015.00177. eCollection 2015. Front Physiol. 2015. PMID: 26106334 Free PMC article.
-
New perspectives in cyclic nucleotide-mediated functions in the CNS: the emerging role of cyclic nucleotide-gated (CNG) channels.Pflugers Arch. 2014 Jul;466(7):1241-57. doi: 10.1007/s00424-013-1373-2. Epub 2013 Oct 19. Pflugers Arch. 2014. PMID: 24142069 Review.
-
Olfactory Dysfunction in Patients With CNGB1-Associated Retinitis Pigmentosa.JAMA Ophthalmol. 2018 Jul 1;136(7):761-769. doi: 10.1001/jamaophthalmol.2018.1621. JAMA Ophthalmol. 2018. PMID: 29800053 Free PMC article.
-
The B3 Subunit of the Cone Cyclic Nucleotide-gated Channel Regulates the Light Responses of Cones and Contributes to the Channel Structural Flexibility.J Biol Chem. 2016 Apr 15;291(16):8721-34. doi: 10.1074/jbc.M115.696138. Epub 2016 Feb 18. J Biol Chem. 2016. PMID: 26893377 Free PMC article.
-
PACS-1 mediates phosphorylation-dependent ciliary trafficking of the cyclic-nucleotide-gated channel in olfactory sensory neurons.J Neurosci. 2009 Aug 26;29(34):10541-51. doi: 10.1523/JNEUROSCI.1590-09.2009. J Neurosci. 2009. PMID: 19710307 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

