The WTM genes in budding yeast amplify expression of the stress-inducible gene RNR3
- PMID: 16980392
- PMCID: PMC1667055
- DOI: 10.1534/genetics.106.062042
The WTM genes in budding yeast amplify expression of the stress-inducible gene RNR3
Abstract
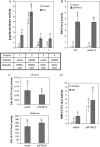
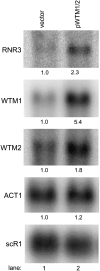
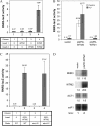
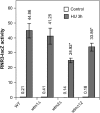
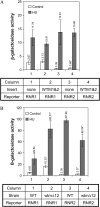
Cellular responses to DNA damage and inhibited replication are evolutionarily conserved sets of pathways that are critical to preserving genome stability. To identify new participants in these responses, we undertook a screen for regulators that, when present on a high-copy vector, alter expression of a DNA damage-inducible RNR3-lacZ reporter construct in Saccharomyces cerevisiae. From this screen we isolated a plasmid encoding two closely related paralogs, WTM1 and WTM2, that greatly increases constitutive expression of RNR3-lacZ. Moderate overexpression of both genes together, or high-level expression of WTM2 alone from a constitutive promoter, upregulates RNR3-lacZ in the absence of DNA damage. Overexpressed, tagged Wtm2p is associated with the RNR3 promoter, indicating that this effect is likely direct. Further investigation reveals that Wtm2p and Wtm1p, previously described as regulators of meiotic gene expression and transcriptional silencing, amplify transcriptional induction of RNR3 in response to replication stress and modulate expression of genes encoding other RNR subunits.
Figures
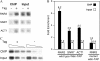
References
-
- Allen, J. B., Z. Zhou, W. Siede, E. C. Friedberg and S. J. Elledge, 1994. The SAD1/RAD53 protein kinase controls multiple checkpoints and DNA damage-induced transcription in yeast. Genes Dev. 8: 2401–2415. - PubMed
-
- Chabes, A., B. Georgieva, V. Domkin, X. Zhao, R. Rothstein et al., 2003. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell 112: 391–401. - PubMed
-
- Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero and P. Hieter, 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110: 119–122. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

