Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development
- PMID: 16980403
- PMCID: PMC1595342
- DOI: 10.1128/EC.00219-06
Production of tyrosol by Candida albicans biofilms and its role in quorum sensing and biofilm development
Abstract
Tyrosol and farnesol are quorum-sensing molecules produced by Candida albicans which accelerate and block, respectively, the morphological transition from yeasts to hyphae. In this study, we have investigated the secretion of tyrosol by C. albicans and explored its likely role in biofilm development. Both planktonic (suspended) cells and biofilms of four C. albicans strains, including three mutants with defined defects in the Efg 1 and Cph 1 morphogenetic signaling pathways, synthesized extracellular tyrosol during growth at 37 degrees C. There was a correlation between tyrosol production and biomass for both cell types. However, biofilm cells secreted at least 50% more tyrosol than did planktonic cells when tyrosol production was related to cell dry weight. The addition of exogenous farnesol to a wild-type strain inhibited biofilm formation by up to 33% after 48 h. Exogenous tyrosol appeared to have no effect, but scanning electron microscopy revealed that tyrosol stimulated hypha production during the early stages (1 to 6 h) of biofilm development. Experiments involving the simultaneous addition of tyrosol and farnesol at different concentrations suggested that the action of farnesol was dominant, and 48-h biofilms formed in the presence of both compounds consisted almost entirely of yeast cells. When biofilm supernatants were tested for their abilities to inhibit or enhance germ tube formation by planktonic cells, the results indicated that tyrosol activity exceeds that of farnesol after 14 h, but not after 24 h, and that farnesol activity increases significantly during the later stages (48 to 72 h) of biofilm development. Overall, our results support the conclusion that tyrosol acts as a quorum-sensing molecule for biofilms as well as for planktonic cells and that its action is most significant during the early and intermediate stages of biofilm formation.
Figures
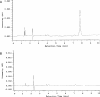

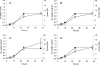
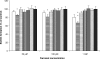
 ), 2 h of biofilm formation (▥), 4 h of biofilm formation (░⃞), and 24 h of biofilm formation (
), 2 h of biofilm formation (▥), 4 h of biofilm formation (░⃞), and 24 h of biofilm formation ( ). ▪, control biofilm (no farnesol added). P was <0.05 (*) and <0.001 (**) for treated biofilms compared with untreated controls (Student's t test).
). ▪, control biofilm (no farnesol added). P was <0.05 (*) and <0.001 (**) for treated biofilms compared with untreated controls (Student's t test).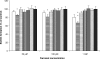
 ), 2 h of biofilm formation (▥), 4 h of biofilm formation (░⃞), and 24 h of biofilm formation (
), 2 h of biofilm formation (▥), 4 h of biofilm formation (░⃞), and 24 h of biofilm formation ( ). ▪, control biofilm (no farnesol added). P was <0.05 (*) and <0.001 (**) for treated biofilms compared with untreated controls (Student's t test).
). ▪, control biofilm (no farnesol added). P was <0.05 (*) and <0.001 (**) for treated biofilms compared with untreated controls (Student's t test).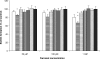
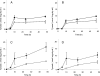
 ), 2 h of biofilm formation (▥), 4 h of biofilm formation (░⃞), and 24 h of biofilm formation (
), 2 h of biofilm formation (▥), 4 h of biofilm formation (░⃞), and 24 h of biofilm formation ( ). ▪, control biofilm (no farnesol added). P was <0.05 (*) and <0.001 (**) for treated biofilms compared with untreated controls (Student's t test).
). ▪, control biofilm (no farnesol added). P was <0.05 (*) and <0.001 (**) for treated biofilms compared with untreated controls (Student's t test).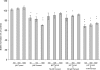


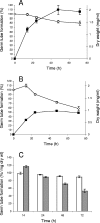
References
-
- Alem, M. A. S., and L. J. Douglas. 2005. Prostaglandin production during growth of Candida albicans biofilms. J. Med. Microbiol. 54:1001-1005. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644-656. - PubMed
-
- Batrakov, S. G., G. I. El'Registan, N. N. Pridachina, V. A. Nenashev, A. N. Kozlova, M. N. Griaznova, and I. N. Zolotareva. 1993. Tyrosol—the autoregulatory d1 factor of the yeast Saccharomyces cerevisiae. Mikrobiologiya 62:633-638. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

