Normal-repeat-length polyglutamine peptides accelerate aggregation nucleation and cytotoxicity of expanded polyglutamine proteins
- PMID: 16980414
- PMCID: PMC1599969
- DOI: 10.1073/pnas.0602348103
Normal-repeat-length polyglutamine peptides accelerate aggregation nucleation and cytotoxicity of expanded polyglutamine proteins
Abstract
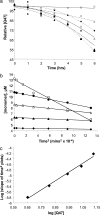
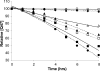
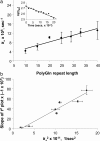
The dependence of disease risk and age-of-onset on expanded CAG repeat length in diseases like Huntington's disease (HD) is well established and correlates with the repeat-length-dependent nucleation kinetics of polyglutamine (polyGln) aggregation. The wide variation in ages of onset among patients with the same repeat length, however, suggests a role for modifying factors. Here we describe the ability of normal-length polyGln repeat sequences to greatly accelerate the nucleation kinetics of an expanded polyGln peptide. We find that normal-length polyGln peptides enhance the in vitro nucleation kinetics of a Q(47) peptide in a concentration-dependent and repeat-length-dependent manner. In vivo, we show that coexpression of a Q(20) sequence in a Drosophila model of HD expressing Htt exon 1 protein with an Q(93) repeat accelerates both aggregate formation and neurotoxicity. The accelerating effect of short polyGln peptides is attributable to the promiscuity of polyGln aggregate elongation and reflects the intimate relationship between nucleus formation and early elongation events in establishing nucleation kinetics. The results suggest that the overall state of the polyGln protein network in a cellular environment may have a profound effect on the toxic consequences of polyGln expansion and thus may serve as a genetic modifier of age of onset in HD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bates GP, Benn C. In: Huntington's Disease. Bates GP, Harper PS, Jones L, editors. Oxford, UK: Oxford Univ Press; 2002. pp. 429–472.
-
- Chen S, Berthelier V, Yang W, Wetzel R. J Mol Biol. 2001;311:173–182. - PubMed
-
- Wetzel R. In: Genetic Instabilities and Neurological Diseases. Wells R, Ashizawa T, editors. San Diego: Elsevier; 2006. pp. 517–534.
-
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Science. 1997;277:1990–1993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

