Revised model for Enterococcus faecalis fsr quorum-sensing system: the small open reading frame fsrD encodes the gelatinase biosynthesis-activating pheromone propeptide corresponding to staphylococcal agrd
- PMID: 16980448
- PMCID: PMC1698201
- DOI: 10.1128/JB.00865-06
Revised model for Enterococcus faecalis fsr quorum-sensing system: the small open reading frame fsrD encodes the gelatinase biosynthesis-activating pheromone propeptide corresponding to staphylococcal agrd
Abstract
Gelatinase biosynthesis-activating pheromone (GBAP) is an autoinducing peptide involved in Enterococcus faecalis fsr quorum sensing, and its 11-amino-acid sequence has been identified in the C-terminal region of the 242-residue deduced fsrB product (J. Nakayama et al., Mol. Microbiol. 41:145-154, 2001). In this study, however, we demonstrated the existence of fsrD, encoding the GBAP propeptide, which is in frame with fsrB but is translated independently of fsrB. It was also demonstrated that FsrB', an FsrD segment-truncated FsrB, functions as a cysteine protease-like processing enzyme to generate GBAP from FsrD. This revised model is consistent with the staphylococcal agr system.
Figures



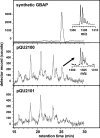

References
-
- Benhar, I., C. Miller, and H. Engelberg-Kulka. 1992. Frameshifting in the expression of the Escherichia coli trpR gene. Mol. Microbiol. 6:2777-2784. - PubMed
-
- Bourgogne, A., S. G. Hilsenbeck, G. M. Dunny, and B. E. Murray. 2006. Comparison of OG1RF and an isogenic fsrB deletion mutant by transcriptional analysis: the Fsr system of Enterococcus faecalis is more than the activator of gelatinase and serine protease. J. Bacteriol. 188:2875-2884. - PMC - PubMed
-
- Cruz-Rodz, A. L., and M. S. Gilmore. 1990. High efficiency introduction of plasmid DNA into glycine treated Enterococcus faecalis by electroporation. Mol. Gen. Genet. 224:152-154. - PubMed
-
- Cserzo, M., E. Wallin, I. Simon, G. von Heijne, and A. Elofsson. 1997. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 10:673-676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

