Phosphorylation of phenol by phenylphosphate synthase: role of histidine phosphate in catalysis
- PMID: 16980461
- PMCID: PMC1636309
- DOI: 10.1128/JB.00785-06
Phosphorylation of phenol by phenylphosphate synthase: role of histidine phosphate in catalysis
Abstract
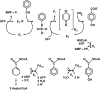
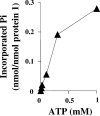
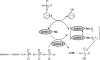
The anaerobic metabolism of phenol proceeds via carboxylation to 4-hydroxybenzoate by a two-step process involving seven proteins and two enzymes ("biological Kolbe-Schmitt carboxylation"). MgATP-dependent phosphorylation of phenol catalyzed by phenylphosphate synthase is followed by phenylphosphate carboxylation. Phenylphosphate synthase shows similarities to phosphoenolpyruvate (PEP) synthase and was studied for the bacterium Thauera aromatica. It consists of three proteins and transfers the beta-phosphoryl from ATP to phenol; the products are phenylphosphate, AMP, and phosphate. We showed that protein 1 becomes phosphorylated in the course of the reaction cycle by [beta-(32)P]ATP. This reaction requires protein 2 and is severalfold stimulated by protein 3. Stimulation of the reaction by 1 M sucrose is probably due to stabilization of the protein(s). Phosphorylated protein 1 transfers the phosphoryl group to phenolic substrates. The primary structure of protein 1 was analyzed by nanoelectrospray mass spectrometry after CNBr cleavage, trypsin digestion, and online high-pressure liquid chromatography at alkaline pH. His-569 was identified as the phosphorylated amino acid. We propose a catalytic ping-pong mechanism similar to that of PEP synthase. First, a diphosphoryl group is transferred to His-569 in protein 1, from which phosphate is cleaved to render the reaction unidirectional. Histidine phosphate subsequently serves as the actual phosphorylation agent.
Figures







References
-
- Anders, H. J., A. Kaetzke, P. Kämpfer, W. Ludwig, and G. Fuchs. 1995. Taxonomic position aromatic-degrading denitrifying pseudomonad strains K 172 and KB 740 and their description as new members of the genera Thauera as Thauera aromatica sp. nov., and Azoarcus, as Azoarcus evansii sp. nov., respectively, members of the Proteobacteria. Int. J. Syst. Bacteriol. 45:327-333. - PubMed
-
- Aresta, M., and A. Dibenedetto. 2002. Development of environmentally friendly syntheses: use of enzymes and biomimetic systems for the direct carboxylation of organic substrates. J. Biotechnol. 90:113-128. - PubMed
-
- Berman, K. M., and M. Cohn. 1970. Phosphoenolpyruvate synthase of Escherichia coli: purification, some properties, and the role of divalent metal ions. J. Biol. Chem. 245:5309-5318. - PubMed
-
- Berman, K. M., and M. Cohn. 1970. Phosphoenolpyruvate synthase: partial reactions studied with adenosine triphosphate analogues and the inorganic phosphate-H218O exchange reaction. J. Biol. Chem. 245:5319-5325. - PubMed
-
- Besant, P. G., and P. V. Attwood. 1998. Problems with phosphoamino acid analysis using alkaline hydrolysis. Anal. Biochem. 265:187-190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

