The atlA operon of Streptococcus mutans: role in autolysin maturation and cell surface biogenesis
- PMID: 16980491
- PMCID: PMC1595523
- DOI: 10.1128/JB.00536-06
The atlA operon of Streptococcus mutans: role in autolysin maturation and cell surface biogenesis
Abstract
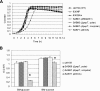
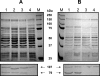
The Smu0630 protein (AtlA) was recently shown to be involved in cell separation, biofilm formation, and autolysis. Here, transcriptional studies revealed that atlA is part of a multigene operon under the control of at least three promoters. The morphology and biofilm-forming capacity of a nonpolar altA mutant could be restored to that of the wild-type strain by adding purified AtlA protein to the medium. A series of truncated derivatives of AtlA revealed that full activity required the C terminus and repeat regions. AtlA was cell associated and readily extractable from with sodium dodecyl sulfate. Of particular interest, the surface protein profile of AtlA-deficient strains was dramatically altered compared to the wild-type strain, as was the nature of the association of the multifunctional adhesin P1 with the cell wall. In addition, AtlA-deficient strains failed to develop competence as effectively as the parental strain. Mutation of thmA, which can be cotranscribed with atlA and encodes a putative pore-forming protein, resulted in a phenotype very similar to that of the AtlA-deficient strain. ThmA was also shown to be required for efficient processing of AtlA to its mature form, and treatment of the thmA mutant strain with full-length AtlA protein did not restore normal cell separation and biofilm formation. The effects of mutating other genes in the operon on cell division, biofilm formation, or AtlA biogenesis were not as profound. This study reveals that AtlA is a surface-associated protein that plays a critical role in the network connecting cell surface biogenesis, biofilm formation, genetic competence, and autolysis.
Figures




References
-
- Alloing, G., B. Martin, C. Granadel, and J. P. Claverys. 1998. Development of competence in Streptococcus pneumoniae: pheromone autoinduction and control of quorum sensing by the oligopeptide permease. Mol. Microbiol. 29:75-83. - PubMed
-
- Azoulay-Dupuis, E., V. Rieux, C. Rivier, and M. C. Trombe. 1998. Pleiotropic mutations alter the kinetics of calcium transport, competence regulation, autolysis and experimental virulence in Streptococcus pneumoniae. Res. Microbiol. 149:5-13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

