Zebra fish Dnmt1 and Suv39h1 regulate organ-specific terminal differentiation during development
- PMID: 16980612
- PMCID: PMC1592902
- DOI: 10.1128/MCB.00312-06
Zebra fish Dnmt1 and Suv39h1 regulate organ-specific terminal differentiation during development
Abstract
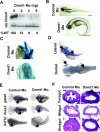
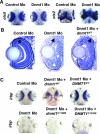
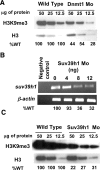
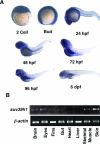
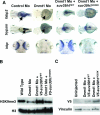
DNA methylation and histone methylation are two key epigenetic modifications that help govern heterochromatin dynamics. The roles for these chromatin-modifying activities in directing tissue-specific development remain largely unknown. To address this issue, we examined the roles of DNA methyltransferase 1 (Dnmt1) and the H3K9 histone methyltransferase Suv39h1 in zebra fish development. Knockdown of Dnmt1 in zebra fish embryos caused defects in terminal differentiation of the intestine, exocrine pancreas, and retina. Interestingly, not all tissues required Dnmt1, as differentiation of the liver and endocrine pancreas appeared normal. Proper differentiation depended on Dnmt1 catalytic activity, as Dnmt1 morphants could be rescued by active zebra fish or human DNMT1 but not by catalytically inactive derivatives. Dnmt1 morphants exhibited dramatic reductions of both genomic cytosine methylation and genome-wide H3K9 trimethyl levels, leading us to investigate the overlap of in vivo functions of Dnmt1 and Suv39h1. Embryos lacking Suv39h1 had organ-specific terminal differentiation defects that produced largely phenocopies of Dnmt1 morphants but retained wild-type levels of DNA methylation. Remarkably, suv39h1 overexpression rescued markers of terminal differentiation in Dnmt1 morphants. Our results suggest that Dnmt1 activity helps direct histone methylation by Suv39h1 and that, together, Dnmt1 and Suv39h1 help guide the terminal differentiation of particular tissues.
Figures


Similar articles
-
Dnmt3 and G9a cooperate for tissue-specific development in zebrafish.J Biol Chem. 2010 Feb 5;285(6):4110-4121. doi: 10.1074/jbc.M109.073676. Epub 2009 Nov 29. J Biol Chem. 2010. PMID: 19946145 Free PMC article.
-
Zfp296 negatively regulates H3K9 methylation in embryonic development as a component of heterochromatin.Sci Rep. 2017 Sep 29;7(1):12462. doi: 10.1038/s41598-017-12772-y. Sci Rep. 2017. PMID: 28963472 Free PMC article.
-
Uhrf1 and Dnmt1 are required for development and maintenance of the zebrafish lens.Dev Biol. 2011 Feb 1;350(1):50-63. doi: 10.1016/j.ydbio.2010.11.009. Epub 2010 Nov 30. Dev Biol. 2011. PMID: 21126517 Free PMC article.
-
Loss of Dnmt1 catalytic activity reveals multiple roles for DNA methylation during pancreas development and regeneration.Dev Biol. 2009 Oct 1;334(1):213-23. doi: 10.1016/j.ydbio.2009.07.017. Epub 2009 Jul 22. Dev Biol. 2009. PMID: 19631206 Free PMC article.
-
The DNA methyltransferases associate with HP1 and the SUV39H1 histone methyltransferase.Nucleic Acids Res. 2003 May 1;31(9):2305-12. doi: 10.1093/nar/gkg332. Nucleic Acids Res. 2003. PMID: 12711675 Free PMC article.
Cited by
-
Zebrafish Discoveries in Cancer Epigenetics.Adv Exp Med Biol. 2016;916:169-97. doi: 10.1007/978-3-319-30654-4_8. Adv Exp Med Biol. 2016. PMID: 27165354 Free PMC article. Review.
-
Silencing DNA methyltransferase 1 (DNMT1) inhibits proliferation, metastasis and invasion in ESCC by suppressing methylation of RASSF1A and DAPK.Oncotarget. 2016 Jul 12;7(28):44129-44141. doi: 10.18632/oncotarget.9866. Oncotarget. 2016. PMID: 27286455 Free PMC article.
-
The zebrafish transcriptome during early development.BMC Dev Biol. 2011 May 24;11:30. doi: 10.1186/1471-213X-11-30. BMC Dev Biol. 2011. PMID: 21609443 Free PMC article.
-
DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45.Cell. 2008 Dec 26;135(7):1201-12. doi: 10.1016/j.cell.2008.11.042. Cell. 2008. PMID: 19109892 Free PMC article.
-
Developmental and environmental epigenetic programming of the endocrine pancreas: consequences for type 2 diabetes.Cell Mol Life Sci. 2013 May;70(9):1575-95. doi: 10.1007/s00018-013-1297-1. Epub 2013 Mar 6. Cell Mol Life Sci. 2013. PMID: 23463236 Free PMC article. Review.
References
-
- Amatruda, J. F., J. L. Shepard, H. M. Stern, and L. I. Zon. 2002. Zebrafish as a cancer model system. Cancer Cell 1:229-231. - PubMed
-
- Biemar, F., F. Argenton, R. Schmidtke, S. Epperlein, B. Peers, and W. Driever. 2001. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev. Biol. 230:189-203. - PubMed
-
- Bird, A. P. 1996. The relationship of DNA methylation to cancer. Cancer Surv. 28:87-101. - PubMed
-
- Davidson, A. J., P. Ernst, Y. Wang, M. P. Dekens, P. D. Kingsley, J. Palis, S. J. Korsmeyer, G. Q. Daley, and L. I. Zon. 2003. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature 425:300-306. - PubMed
-
- Denovan-Wright, E. M., M. Pierce, M. K. Sharma, and J. M. Wright. 2000. cDNA sequence and tissue-specific expression of a basic liver-type fatty acid binding protein in adult zebrafish (Danio rerio). Biochim. Biophys. Acta 1492:227-232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
