Hematopoietic precursor cells transiently reestablish permissiveness for X inactivation
- PMID: 16980619
- PMCID: PMC1592878
- DOI: 10.1128/MCB.00810-06
Hematopoietic precursor cells transiently reestablish permissiveness for X inactivation
Abstract
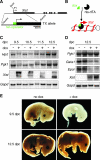
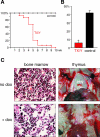
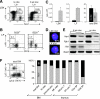
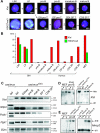
Xist is the trigger for X inactivation in female mammals. The long noncoding Xist RNA localizes along one of the two female X chromosomes and initiates chromosome-wide silencing in the early embryo. In differentiated cells, Xist becomes dispensable for the maintenance of the inactive X, and its function for initiation of silencing is lost. How Xist mediates gene repression remains an open question. Here, we use an inducible Xist allele in adult mice to identify cells in which Xist can cause chromosome-wide silencing. We show that Xist has the ability to initiate silencing in immature hematopoietic precursor cells. In contrast, hematopoietic stem cells and mature blood cells are unable to initiate ectopic X inactivation. This indicates that pathways critical for silencing are transiently activated in hematopoietic differentiation. Xist-responsive cell types in normal female mice show a change of chromatin marks on the inactive X. However, dosage compensation is maintained throughout hematopoiesis. Therefore, Xist can initiate silencing in precursors with concomitant maintenance of dosage compensation. This suggests that Xist function is restricted in development by the limited activity of epigenetic pathways rather than by a change in the responsiveness of chromatin between embryonic and differentiated cell types.
Figures

References
-
- Bacher, C. P., M. Guggiari, B. Brors, S. Augui, P. Clerc, P. Avner, R. Eils, and E. Heard. 2006. Transient colocalization of X-inactivation centres accompanies the initiation of X inactivation. Nat. Cell. Biol. - PubMed
-
- Balciunaite, G., R. Ceredig, S. Massa, and A. G. Rolink. 2005. A B220+ CD117+ CD19− hematopoietic progenitor with potent lymphoid andmyeloid developmental potential. Eur. J. Immunol. 2019-2030. - PubMed
-
- Bassing, C. H., W. Swat, and F. W. Alt. 2002. The mechanism and regulation of chromosomal V(D)J recombination. Cell 109(Suppl.):S45-S55. - PubMed
-
- Borsani, G., R. Tonlorenzi, M. C. Simmler, L. Dandolo, D. Arnaud, V. Capra, M. Grompe, A. Pizzuti, D. Muzny, C. Lawrence, et al. 1991. Characterization of a murine gene expressed from the inactive X chromosome. Nature 351:325-329. - PubMed
-
- Brockdorff, N. 2002. X-chromosome inactivation: closing in on proteins that bind Xist RNA. Trends Genet. 18:352-358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
