Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1
- PMID: 16980963
- PMCID: PMC1997306
- DOI: 10.1038/nn1775
Asymmetrical beta-actin mRNA translation in growth cones mediates attractive turning to netrin-1
Abstract
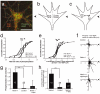
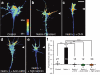
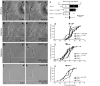
Local protein synthesis regulates the turning of growth cones to guidance cues, yet little is known about which proteins are synthesized or how they contribute to directional steering. Here we show that beta-actin mRNA resides in Xenopus laevis retinal growth cones where it binds to the RNA-binding protein Vg1RBP. Netrin-1 induces the movement of Vg1RBP granules into filopodia, suggesting that it may direct the localization and translation of mRNAs in growth cones. Indeed, a gradient of netrin-1 activates a translation initiation regulator, eIF-4E-binding protein 1 (4EBP), asymmetrically and triggers a polarized increase in beta-actin translation on the near side of the growth cone before growth cone turning. Inhibition of beta-actin translation abolishes both the asymmetric rise in beta-actin and attractive, but not repulsive, turning. Our data suggest that newly synthesized beta-actin, concentrated near sites of signal reception, provides the directional bias for polymerizing actin in the direction of an attractive stimulus.
Figures
Comment in
-
Turning by asymmetric actin.Nat Neurosci. 2006 Oct;9(10):1201-3. doi: 10.1038/nn1006-1201. Nat Neurosci. 2006. PMID: 17001333 No abstract available.
References
-
- Guirland C, Suzuki S, Kojima M, Lu B, Zheng JQ. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. - PubMed
-
- Wen Z, Guirland C, Ming GL, Zheng JQ. A CaMKII/calcineurin switch controls the direction of Ca(2+)-dependent growth cone guidance. Neuron. 2004;43:835–46. - PubMed
-
- Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403:89–93. - PubMed
-
- Li Y, et al. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

