Cholinergic regulation of epithelial ion transport in the mammalian intestine
- PMID: 16981004
- PMCID: PMC2014671
- DOI: 10.1038/sj.bjp.0706889
Cholinergic regulation of epithelial ion transport in the mammalian intestine
Abstract
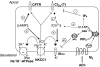
Acetylcholine (ACh) is critical in controlling epithelial ion transport and hence water movements for gut hydration. Here we review the mechanism of cholinergic control of epithelial ion transport across the mammalian intestine. The cholinergic nervous system affects basal ion flux and can evoke increased active ion transport events. Most studies rely on measuring increases in short-circuit current (ISC = active ion transport) evoked by adding ACh or cholinomimetics to intestinal tissue mounted in Ussing chambers. Despite subtle species and gut regional differences, most data indicate that, under normal circumstances, the effect of ACh on intestinal ion transport is mainly an increase in Cl- secretion due to interaction with epithelial M3 muscarinic ACh receptors (mAChRs) and, to a lesser extent, neuronal M1 mAChRs; however, AChR pharmacology has been plagued by a lack of good receptor subtype-selective compounds. Mice lacking M3 mAChRs display intact cholinergically-mediated intestinal ion transport, suggesting a possible compensatory mechanism. Inflamed tissues often display perturbations in the enteric cholinergic system and reduced intestinal ion transport responses to cholinomimetics. The mechanism(s) underlying this hyporesponsiveness are not fully defined. Inflammation-evoked loss of mAChR-mediated control of epithelial ion transport in the mouse reveals a role for neuronal nicotinic AChRs, representing a hitherto unappreciated braking system to limit ACh-evoked Cl- secretion. We suggest that: i) pharmacological analyses should be supported by the use of more selective compounds and supplemented with molecular biology techniques targeting specific ACh receptors and signalling molecules, and ii) assessment of ion transport in normal tissue must be complemented with investigations of tissues from patients or animals with intestinal disease to reveal control mechanisms that may go undetected by focusing on healthy tissue only.
Figures

References
-
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol. 1992;41:802–808. - PubMed
-
- Anini Y, Brubaker PL. Muscarinic receptors control glucagon-like peptide 1 secretion by human endocrine L cells. Endocrinology. 2003;144:3244–3250. - PubMed
-
- Anini Y, Hansotia T, Brubaker PL. Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology. 2002;143:2420–2426. - PubMed
-
- Argenzio RA, Armstrong M, Rhoads JM. Role of the enteric nervous system in piglet cryptosporidiosis. J Pharmacol Exp Ther. 1996;279:1109–1115. - PubMed
-
- Asfaha S, Bell CJ, Wallace JL, MacNaughton WK. Prolonged colonic epithelial hyporesponsiveness after colitis: role of inducible nitric oxide synthase. Am J Physiol. 1999;276:G703–G710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

