Reaction of adenosylcobalamin-dependent glutamate mutase with 2-thiolglutarate
- PMID: 16981724
- PMCID: PMC2517135
- DOI: 10.1021/bi061067n
Reaction of adenosylcobalamin-dependent glutamate mutase with 2-thiolglutarate
Abstract
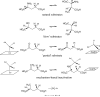
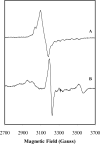
We have investigated the reaction of glutamate mutase with the glutamate analogue, 2-thiolglutarate. In the standard assay, 2-thiolglutarate behaves as a competitive inhibitor with a Ki of 0.05 mM. However, rather than simply binding inertly at the active site, 2-thiolglutarate elicits cobalt-carbon bond homolysis and the formation of 5'-deoxyadenosine. The enzyme exhibits a complicated EPR spectrum in the presence of 2-thiolglutarate that is markedly different from any previously observed with the enzyme. The spectrum was simulated well by assuming that it arises from electron-electron spin coupling between a thioglycolyl radical and low-spin Co2+ in cob(II)alamin. Analysis of the zero-field splitting parameters obtained from the simulations places the organic radical approximately 10 A from the cobalt and at a tilt angle of approximately 70 degrees to the normal of the corrin ring. This orientation is in good agreement with that expected from the crystal structure of glutamate mutase complexed with the substrate. 2-Thiolglutarate appears to react in a manner analogous to that of glutamate by first forming a thiolglutaryl radical at C-4 that then undergoes fragmentation to produce acrylate and the sulfur-stabilized thioglycolyl radical. The thioglycolyl radical accumulates on the enzyme, suggesting it is too stable to undergo further steps in the mechanism at a detectable rate.
Figures



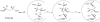
References
-
- Toraya T. Radical catalysis in coenzyme B12-dependent isomerization (eliminating) reactions. Chem. Rev. 2003;103:2095–2127. - PubMed
-
- Banerjee R, Ragsdale SW. The many faces of vitamin B12: Catalysis by cobalamin-dependent enzymes. Ann. Rev. Biochem. 2003;72:209–247. - PubMed
-
- Banerjee R. Radical carbon skeleton rearrangements: Catalysis by coenzyme B12-dependent mutases. Chem. Rev. 2003;103:2083–2094. - PubMed
-
- Marsh ENG, Drennan CL. Adenosylcobalamin-dependent isomerases: new insights into structure and mechanism. Curr. Opin. Chem. Biol. 2001;5:499–505. - PubMed
-
- Banerjee R. Radical Peregrinations Catalyzed by Coenzyme B12-Dependent Enzymes. Biochemistry. 2001;40:6191–96198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

