Controlling activation of the RNA-dependent protein kinase by siRNAs using site-specific chemical modification
- PMID: 16982647
- PMCID: PMC1635244
- DOI: 10.1093/nar/gkl464
Controlling activation of the RNA-dependent protein kinase by siRNAs using site-specific chemical modification
Abstract
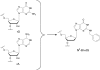
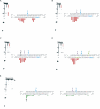
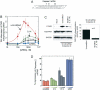
The RNA-dependent protein kinase (PKR) is activated by binding to double-stranded RNA (dsRNA). Activation of PKR by short-interfering RNAs (siRNAs) and stimulation of the innate immune response has been suggested to explain certain off-target effects in some RNA interference experiments. Here we show that PKR's kinase activity is stimulated in vitro 3- to 5-fold by siRNA duplexes with 19 bp and 2 nt 3'-overhangs, whereas the maximum activation observed for poly(I)*poly(C) was 17-fold over background under the same conditions. Directed hydroxyl radical cleavage experiments indicated that siRNA duplexes have at least four different binding sites for PKR's dsRNA binding motifs (dsRBMs). The location of these binding sites suggested specific nucleotide positions in the siRNA sense strand that could be modified with a corresponding loss of PKR binding. Modification at these sites with N2-benzyl-2'-deoxyguanosine (BndG) blocked interaction with PKR's dsRBMs and inhibited activation of PKR by the siRNA. Importantly, modification of an siRNA duplex that greatly reduced PKR activation did not prevent the duplex from lowering mRNA levels of a targeted message by RNA interference in HeLa cells. Thus, these studies demonstrate that specific positions in an siRNA can be rationally modified to prevent interaction with components of cellular dsRNA-regulated pathways.
Figures
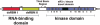

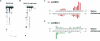


Similar articles
-
Identification of binding sites for both dsRBMs of PKR on kinase-activating and kinase-inhibiting RNA ligands.Biochemistry. 2002 Apr 9;41(14):4511-20. doi: 10.1021/bi0120594. Biochemistry. 2002. PMID: 11926812
-
Selective binding by the RNA binding domain of PKR revealed by affinity cleavage.Biochemistry. 2001 Apr 10;40(14):4272-80. doi: 10.1021/bi002512w. Biochemistry. 2001. PMID: 11284683
-
Characterization of the direct physical interaction of nc886, a cellular non-coding RNA, and PKR.FEBS Lett. 2012 Sep 21;586(19):3477-84. doi: 10.1016/j.febslet.2012.07.076. Epub 2012 Aug 9. FEBS Lett. 2012. PMID: 22986343
-
Discriminating Self and Non-Self by RNA: Roles for RNA Structure, Misfolding, and Modification in Regulating the Innate Immune Sensor PKR.Acc Chem Res. 2016 Jun 21;49(6):1242-9. doi: 10.1021/acs.accounts.6b00151. Epub 2016 Jun 8. Acc Chem Res. 2016. PMID: 27269119 Free PMC article. Review.
-
The search for a PKR code-differential regulation of protein kinase R activity by diverse RNA and protein regulators.RNA. 2019 May;25(5):539-556. doi: 10.1261/rna.070169.118. Epub 2019 Feb 15. RNA. 2019. PMID: 30770398 Free PMC article. Review.
Cited by
-
Therapeutic siRNA: State-of-the-Art and Future Perspectives.BioDrugs. 2022 Sep;36(5):549-571. doi: 10.1007/s40259-022-00549-3. Epub 2022 Aug 23. BioDrugs. 2022. PMID: 35997897 Free PMC article. Review.
-
Native tertiary structure and nucleoside modifications suppress tRNA's intrinsic ability to activate the innate immune sensor PKR.PLoS One. 2013;8(3):e57905. doi: 10.1371/journal.pone.0057905. Epub 2013 Mar 4. PLoS One. 2013. PMID: 23483938 Free PMC article.
-
Current Development of siRNA Bioconjugates: From Research to the Clinic.Front Pharmacol. 2019 Apr 26;10:444. doi: 10.3389/fphar.2019.00444. eCollection 2019. Front Pharmacol. 2019. PMID: 31105570 Free PMC article. Review.
-
Molecular Mechanism of the Antiproliferative Activity of Short Immunostimulating dsRNA.Front Oncol. 2019 Dec 20;9:1454. doi: 10.3389/fonc.2019.01454. eCollection 2019. Front Oncol. 2019. PMID: 31921696 Free PMC article.
-
BRAFV600 and ErbB inhibitors directly activate GCN2 in an off-target manner to limit cancer cell proliferation.bioRxiv [Preprint]. 2024 Dec 20:2024.12.19.629301. doi: 10.1101/2024.12.19.629301. bioRxiv. 2024. PMID: 39763857 Free PMC article. Preprint.
References
-
- Kaufman R. The double-stranded RNA-activated protein kinase PKR. In: Sonenberg N., Hershey J.W.B., Mathews M.B., editors. Translation Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2000. pp. 503–527.
-
- Galabru J., Hovanessian A. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J. Biol. Chem. 1987;262:15538–15544. - PubMed
-
- Koromilas A.E., Roy S., Barber G.N., Katze M.G., Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science. 1992;257:1685–1689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

