Mice mutant in the DM domain gene Dmrt4 are viable and fertile but have polyovular follicles
- PMID: 16982677
- PMCID: PMC1636805
- DOI: 10.1128/MCB.00959-06
Mice mutant in the DM domain gene Dmrt4 are viable and fertile but have polyovular follicles
Abstract
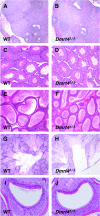
Proteins containing the DM domain, a zinc finger-like DNA binding motif, have been implicated in sexual differentiation in diverse metazoan organisms. Of seven mammalian DM domain genes, only Dmrt1 and Dmrt2 have been functionally analyzed. Here, we report expression analysis and targeted disruption of Dmrt4 (also called DmrtA1) in the mouse. Dmrt4 is widely expressed during embryonic and postnatal development. However, we find that mice homozygous for a putative null mutation in Dmrt4 develop essentially normally, undergo full sexual differentiation in both sexes, and are fertile. We observed two potential mutant phenotypes in Dmrt4 mutant mice. First, ovaries of most mutant females have polyovular follicles, suggesting a role in folliculogenesis. Second, 25% of mutant males consistently exhibited copulatory behavior toward other males. We also tested potential redundancy between Dmrt4 and two other gonadally expressed DM domain genes, Dmrt1 and Dmrt7. We observed no enhancement of gonadal phenotypes in the double mutants, suggesting that these genes function independently in gonadal development.
Figures






References
-
- Coschigano, K. T., and P. C. Wensink. 1993. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 7:42-54. - PubMed
-
- Dominguez-Salazar, E., H. L. Bateman, and E. F. Rissman. 2004. Background matters: the effects of estrogen receptor alpha gene disruption on male sexual behavior are modified by background strain. Horm. Behav. 46:482-490. - PubMed
-
- Hogan, B., R. Beddington, F. Constantini, and E. Laci. 1994. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
