The middle domain of Hsp90 acts as a discriminator between different types of client proteins
- PMID: 16982694
- PMCID: PMC1636778
- DOI: 10.1128/MCB.02188-05
The middle domain of Hsp90 acts as a discriminator between different types of client proteins
Abstract
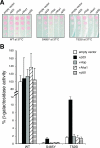
The mechanism of client protein activation by Hsp90 is enigmatic, and it is uncertain whether Hsp90 employs a common route for all proteins. Using a mutational analysis approach, we investigated the activation of two types of client proteins, glucocorticoid receptor (GR) and the kinase v-Src by the middle domain of Hsp90 (Hsp90M) in vivo. Remarkably, the overall cellular activity of v-Src was highly elevated in a W300A mutant yeast strain due to a 10-fold increase in cellular protein levels of the kinase. In contrast, the cellular activity of GR remained almost unaffected by the W300A mutation but was dramatically sensitive to S485Y and T525I exchanges. In addition, we show that mutations S485Y and T525I in Hsp90M reduce the ATP hydrolysis rate, suggesting that Hsp90 ATPase is more tightly regulated than assumed previously. Therefore, the activation of GR and v-Src has various demands on Hsp90 biochemistry and is dependent on separate functional regions of Hsp90M. Thus, Hsp90M seems to discriminate between different substrate types and to adjust the molecular chaperone for proper substrate activation.
Figures


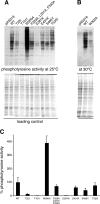
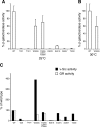
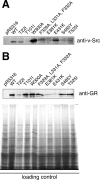
Similar articles
-
Stimulation of the weak ATPase activity of human hsp90 by a client protein.J Mol Biol. 2002 Jan 25;315(4):787-98. doi: 10.1006/jmbi.2001.5245. J Mol Biol. 2002. PMID: 11812147
-
Cofactor Tpr2 combines two TPR domains and a J domain to regulate the Hsp70/Hsp90 chaperone system.EMBO J. 2003 Jul 15;22(14):3613-23. doi: 10.1093/emboj/cdg362. EMBO J. 2003. PMID: 12853476 Free PMC article.
-
A mutation in the catalytic loop of Hsp90 specifically impairs ATPase stimulation by Aha1p, but not Hch1p.J Mol Biol. 2014 Jun 12;426(12):2379-92. doi: 10.1016/j.jmb.2014.04.002. Epub 2014 Apr 12. J Mol Biol. 2014. PMID: 24726918
-
Structure and mechanism of the Hsp90 molecular chaperone machinery.Annu Rev Biochem. 2006;75:271-94. doi: 10.1146/annurev.biochem.75.103004.142738. Annu Rev Biochem. 2006. PMID: 16756493 Review.
-
Advances towards Understanding the Mechanism of Action of the Hsp90 Complex.Biomolecules. 2022 Apr 19;12(5):600. doi: 10.3390/biom12050600. Biomolecules. 2022. PMID: 35625528 Free PMC article. Review.
Cited by
-
Low resolution structural studies indicate that the activator of Hsp90 ATPase 1 (Aha1) of Leishmania braziliensis has an elongated shape which allows its interaction with both N- and M-domains of Hsp90.PLoS One. 2013 Jun 24;8(6):e66822. doi: 10.1371/journal.pone.0066822. Print 2013. PLoS One. 2013. PMID: 23826147 Free PMC article.
-
The conserved arginine 380 of Hsp90 is not a catalytic residue, but stabilizes the closed conformation required for ATP hydrolysis.Protein Sci. 2012 Aug;21(8):1162-71. doi: 10.1002/pro.2103. Protein Sci. 2012. PMID: 22653663 Free PMC article.
-
Activation of autophagy depends on Atg1/Ulk1-mediated phosphorylation and inhibition of the Hsp90 chaperone machinery.Cell Rep. 2023 Jul 25;42(7):112807. doi: 10.1016/j.celrep.2023.112807. Epub 2023 Jul 14. Cell Rep. 2023. PMID: 37453059 Free PMC article.
-
A primate specific extra domain in the molecular chaperone Hsp90.PLoS One. 2013 Aug 9;8(8):e71856. doi: 10.1371/journal.pone.0071856. eCollection 2013. PLoS One. 2013. PMID: 23951259 Free PMC article.
-
Structure, Function, and Regulation of the Hsp90 Machinery.Cold Spring Harb Perspect Biol. 2019 Sep 3;11(9):a034017. doi: 10.1101/cshperspect.a034017. Cold Spring Harb Perspect Biol. 2019. PMID: 30745292 Free PMC article. Review.
References
-
- Dittmar, K. D., D. R. Demady, L. F. Stancato, P. Krishan, and W. B. Pratt. 1997. Folding of the glucocorticoid receptor by heat shock protein (hsp) 90-based chaperone machinery. J. Biol. Chem. 272:21213-21220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
