All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients
- PMID: 16982775
- PMCID: PMC1586106
- DOI: 10.1158/0008-5472.CAN-06-1690
All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients
Abstract
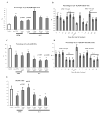
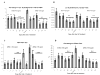
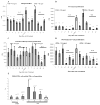
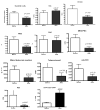
Abnormal dendritic cell differentiation and accumulation of immature myeloid suppressor cells (ImC) is one of the major mechanisms of tumor escape. We tested the possibility of pharmacologic regulation of myeloid cell differentiation using all-trans-retinoic acid (ATRA). Eighteen patients with metastatic renal cell carcinoma were treated with ATRA followed by s.c. interleukin 2 (IL-2). Eight healthy individuals comprised a control group. As expected, the cancer patients had substantially elevated levels of ImC. We observed that ATRA dramatically reduced the number of ImC. This effect was observed only in patients with high plasma concentration of ATRA (>150 ng/mL), but not in patients with lower ATRA concentrations (<135 ng/mL). Effects of ATRA on the proportions of different dendritic cell populations were minor. However, ATRA significantly improved myeloid/lymphoid dendritic cell ratio and the ability of patients' mononuclear cells to stimulate allogeneic T cells. This effect was associated with significant improvement of tetanus-toxoid-specific T-cell response. During the IL-2 treatment, the ATRA effect was completely eliminated. To assess the role of IL-2, specimens from 15 patients with metastatic renal cell carcinoma who had been treated with i.v. IL-2 alone were analyzed. In this group also, IL-2 significantly reduced the number and function of dendritic cells as well as T-cell function. These data indicate that ATRA at effective concentrations eliminated ImC, improved myeloid/lymphoid dendritic cell ratio, dendritic cell function, and antigen-specific T-cell response. ATRA treatment did not result in significant toxicity and it could be tested in therapeutic combination with cancer vaccines.
Figures

References
-
- Gabrilovich D, Pisarev V. Tumor escape from immune response: mechanisms and targets of activity. Curr Drug Targets. 2003;4:525–36. - PubMed
-
- Gabrilovich D. The mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–52. - PubMed
-
- Subiza J, Vinuela J, Rodriguez R, De la Concha E. Development of splenic natural suppressor (NS) cells in Ehrlich tumor-bearing mice. Int J Cancer. 1989;44:307–14. - PubMed
-
- Kusmartsev S, Li Y, Chen S-H. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

