Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA
- PMID: 16982800
- PMCID: PMC2064390
- DOI: 10.1083/jcb.200604069
Cytopathic effects of the cytomegalovirus-encoded apoptosis inhibitory protein vMIA
Abstract
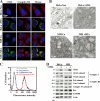
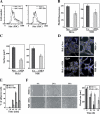
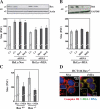
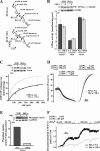
Replication of human cytomegalovirus (CMV) requires the expression of the viral mitochondria-localized inhibitor of apoptosis (vMIA). vMIA inhibits apoptosis by recruiting Bax to mitochondria, resulting in its neutralization. We show that vMIA decreases cell size, reduces actin polymerization, and induces cell rounding. As compared with vMIA-expressing CMV, vMIA-deficient CMV, which replicates in fibroblasts expressing the adenoviral apoptosis suppressor E1B19K, induces less cytopathic effects. These vMIA effects can be separated from its cell death-inhibitory function because vMIA modulates cellular morphology in Bax-deficient cells. Expression of vMIA coincided with a reduction in the cellular adenosine triphosphate (ATP) level. vMIA selectively inhibited one component of the ATP synthasome, namely, the mitochondrial phosphate carrier. Exposure of cells to inhibitors of oxidative phosphorylation produced similar effects, such as an ATP level reduced by 30%, smaller cell size, and deficient actin polymerization. Similarly, knockdown of the phosphate carrier reduced cell size. Our data suggest that the cytopathic effect of CMV can be explained by vMIA effects on mitochondrial bioenergetics.
Figures
Similar articles
-
Structure-function analysis of the interaction between Bax and the cytomegalovirus-encoded protein vMIA.Oncogene. 2007 Nov 1;26(50):7067-80. doi: 10.1038/sj.onc.1210511. Epub 2007 May 14. Oncogene. 2007. PMID: 17496930
-
vMIA, a viral inhibitor of apoptosis targeting mitochondria.Biochimie. 2002 Feb-Mar;84(2-3):177-85. doi: 10.1016/s0300-9084(02)01367-6. Biochimie. 2002. PMID: 12022948 Review.
-
Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria.Proc Natl Acad Sci U S A. 2004 May 25;101(21):7988-93. doi: 10.1073/pnas.0401897101. Epub 2004 May 17. Proc Natl Acad Sci U S A. 2004. PMID: 15148411 Free PMC article.
-
Cytomegaloviruses inhibit Bak- and Bax-mediated apoptosis with two separate viral proteins.Cell Death Differ. 2010 Apr;17(4):655-65. doi: 10.1038/cdd.2009.147. Epub 2009 Oct 9. Cell Death Differ. 2010. PMID: 19816509
-
Cell death suppression by cytomegaloviruses.Apoptosis. 2005 Mar;10(2):251-65. doi: 10.1007/s10495-005-0800-z. Apoptosis. 2005. PMID: 15843887 Review.
Cited by
-
Mitochondrial dynamics and viral infections: A close nexus.Biochim Biophys Acta. 2015 Oct;1853(10 Pt B):2822-33. doi: 10.1016/j.bbamcr.2014.12.040. Epub 2015 Jan 13. Biochim Biophys Acta. 2015. PMID: 25595529 Free PMC article. Review.
-
Viral product trafficking to mitochondria, mechanisms and roles in pathogenesis.Infect Disord Drug Targets. 2012 Feb;12(1):18-37. doi: 10.2174/187152612798994948. Infect Disord Drug Targets. 2012. PMID: 22034933 Free PMC article. Review.
-
Human Cytomegalovirus Alters Host Cell Mitochondrial Function during Acute Infection.J Virol. 2020 Jan 6;94(2):e01183-19. doi: 10.1128/JVI.01183-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31694945 Free PMC article.
-
Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection.Mol Cell Proteomics. 2011 Oct;10(10):M111.009936. doi: 10.1074/mcp.M111.009936. Epub 2011 Jul 8. Mol Cell Proteomics. 2011. PMID: 21742798 Free PMC article.
-
Human cytomegalovirus pUL37x1-induced calcium flux activates PKCα, inducing altered cell shape and accumulation of cytoplasmic vesicles.Proc Natl Acad Sci U S A. 2014 Mar 25;111(12):E1140-8. doi: 10.1073/pnas.1402515111. Epub 2014 Mar 10. Proc Natl Acad Sci U S A. 2014. PMID: 24616524 Free PMC article.
References
-
- Arnoult, D., L.M. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P.U. Park, J. Sharpe, R.J. Youle, and V.S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA. 101:7988–7989. - PMC - PubMed
-
- Atkinson, S.J., M.A. Hosford, and B.A. Molitoris. 2004. Mechanism of actin polymerization in cellular ATP depletion. J. Biol. Chem. 279:5194–5199. - PubMed
-
- Belzacq, A.S., C. El Hamel, H.L.A. Vieira, I. Cohen, D. Haouzi, D. Metivier, P. Marchetti, V. Goldmacher, C. Brenner, and G. Kroemer. 2001. The adenine nucleotide translocator mediates the mitochondrial membrane permeabilization induced by lonidamine, arsenite and CD437. Oncogene. 20:7579–7587. - PubMed
-
- Belzacq, A.S., H.L. Vieira, F. Verrier, G. Vandecasteele, I. Cohen, M.C. Prevost, P.X. Petit, A. Kahn, B. Roques, R. Rizzuto, et al. 2003. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res. 63:541–546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

