Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes
- PMID: 16982801
- PMCID: PMC2064391
- DOI: 10.1083/jcb.200605004
Ca2+ and synaptotagmin VII-dependent delivery of lysosomal membrane to nascent phagosomes
Abstract
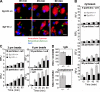
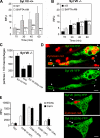
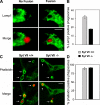
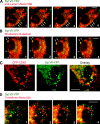
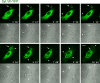
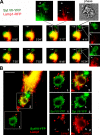
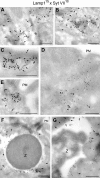
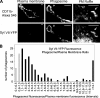
Synaptotagmin (Syt) VII is a ubiquitously expressed member of the Syt family of Ca2+ sensors. It is present on lysosomes in several cell types, where it regulates Ca2+-dependent exocytosis. Because [Ca2+]i and exocytosis have been associated with phagocytosis, we investigated the phagocytic ability of macrophages from Syt VII-/- mice. Syt VII-/- macrophages phagocytose normally at low particle/cell ratios but show a progressive inhibition in particle uptake under high load conditions. Complementation with Syt VII rescues this phenotype, but only when functional Ca2+-binding sites are retained. Reinforcing a role for Syt VII in Ca2+-dependent phagocytosis, particle uptake in Syt VII-/- macrophages is significantly less dependent on [Ca2+]i. Syt VII is concentrated on peripheral domains of lysosomal compartments, from where it is recruited to nascent phagosomes. Syt VII recruitment is rapidly followed by the delivery of Lamp1 to phagosomes, a process that is inhibited in Syt VII-/- macrophages. Thus, Syt VII regulates the Ca2+-dependent mobilization of lysosomes as a supplemental source of membrane during phagocytosis.
Figures
References
-
- Aderem, A., and D.M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593–623. - PubMed
-
- Advani, R.J., H.R. Bae, J.B. Bock, D.S. Chao, Y.C. Doung, R. Prekeris, J.S. Yoo, and R.H. Scheller. 1998. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 273:10317–10324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

