HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells
- PMID: 16982804
- PMCID: PMC2064396
- DOI: 10.1083/jcb.200605113
HDAC3 is crucial in shear- and VEGF-induced stem cell differentiation toward endothelial cells
Abstract
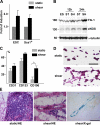
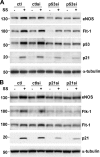
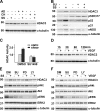
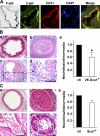
Reendothelialization involves endothelial progenitor cell (EPC) homing, proliferation, and differentiation, which may be influenced by fluid shear stress and local flow pattern. This study aims to elucidate the role of laminar flow on embryonic stem (ES) cell differentiation and the underlying mechanism. We demonstrated that laminar flow enhanced ES cell-derived progenitor cell proliferation and differentiation into endothelial cells (ECs). Laminar flow stabilized and activated histone deacetylase 3 (HDAC3) through the Flk-1-PI3K-Akt pathway, which in turn deacetylated p53, leading to p21 activation. A similar signal pathway was detected in vascular endothelial growth factor-induced EC differentiation. HDAC3 and p21 were detected in blood vessels during embryogenesis. Local transfer of ES cell-derived EPC incorporated into injured femoral artery and reduced neointima formation in a mouse model. These data suggest that shear stress is a key regulator for stem cell differentiation into EC, especially in EPC differentiation, which can be used for vascular repair, and that the Flk-1-PI3K-Akt-HDAC3-p53-p21 pathway is crucial in such a process.
Figures




References
-
- Akimoto, S., M. Mitsumata, T. Sasaguri, and Y. Yoshida. 2000. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21(Sdi1/Cip1/Waf1). Circ. Res. 86:185–190. - PubMed
-
- Bruhl, T., C. Heeschen, A. Aicher, A.S. Jadidi, J. Haendeler, J. Hoffmann, M.D. Schneider, A.M. Zeiher, S. Dimmeler, and L. Rossig. 2004. p21Cip1 levels differentially regulate turnover of mature endothelial cells, endothelial progenitor cells, and in vivo neovascularization. Circ. Res. 94:686–692. - PubMed
-
- Claesson-Welsh, L. 2003. Signal transduction by vascular endothelial growth factor receptors. Biochem. Soc. Trans. 31:20–24. - PubMed
-
- Coultas, L., K. Chawengsaksophak, and J. Rossant. 2005. Endothelial cells and VEGF in vascular development. Nature. 438:937–945. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

