CXCL10/gamma interferon-inducible protein 10-mediated protection against Leishmania amazonensis infection in mice
- PMID: 16982826
- PMCID: PMC1698098
- DOI: 10.1128/IAI.01073-06
CXCL10/gamma interferon-inducible protein 10-mediated protection against Leishmania amazonensis infection in mice
Abstract
Leishmania amazonensis can cause progressive disease in most inbred strains of mice. We have previously shown that L. amazonensis-infected C57BL/6 mice have profound impairments in expression of proinflammatory cytokines and chemokines and in activation of antigen-specific CD4(+) T cells. These impairments are independent of interleukin-4 (IL-4) but partially due to IL-10 production. The precise mechanism of pathogenesis associated with L. amazonensis infection remains largely unresolved. Since chemokines are essential mediators of leukocyte recruitment and effector cell function, we hypothesized that these molecules are important for the initiation of early responses locally and for the eventual control of the infection. In this study, we examined the roles of CXCL10/gamma interferon-inducible protein 10 (IP-10) and CCL2/monocyte chemoattractant protein 1 (MCP-1) in the activation of the macrophage effector function in vitro and their efficacy in ameliorating infection in vivo. Bone marrow-derived macrophages of both BALB/c and C57BL/6 mice were treated with increasing concentrations of recombinant chemokines prior to infection with either stationary-phase promastigotes or tissue-derived amastigotes. We found that treatment with IP-10 or MCP-1 significantly reduced parasite burdens, in a dose-dependent manner, and triggered nitric oxide production. When susceptible C57BL/6 mice were injected locally with IP-10 following L. amazonensis infection, there was a significant delay in lesion development and a reduction in parasite burdens, accompanied by 7- and 3.5-fold increases in gamma interferon and IL-12 secretion, respectively, in restimulated lymph node cells. This study confirms that IP-10 plays a protective role in promoting the reduction of intracellular parasites and thereby opens new avenues for therapeutic control of nonhealing cutaneous leishmaniasis in the New World.
Figures
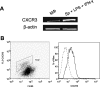
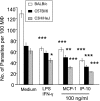
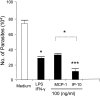
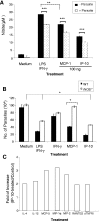
 , P < 0.05;
, P < 0.05; 
 , P < 0.01);
, P < 0.01); 

 , P < 0.001). The data shown are representative of three independent repeats. (C) BM-Mφs of BALB/c mice were seeded into six-well plates (1 × 106 cells/well) and infected with 8 × 106 stationary-phase promastigotes (8:1 parasite-to-cell ratio). At 48 h postinfection, cell-free supernatants were collected for the measurement of cytokine profiles via protein cytokine arrays. The intensities of protein spots for the IP-10-treated group were compared with those of the corresponding spots for the untreated controls, and data are presented as x-fold increases above the infection control levels. The data shown are the results for those molecules that displayed ≥2-fold increases over the infection control levels and are representative of three independent repeats.
, P < 0.001). The data shown are representative of three independent repeats. (C) BM-Mφs of BALB/c mice were seeded into six-well plates (1 × 106 cells/well) and infected with 8 × 106 stationary-phase promastigotes (8:1 parasite-to-cell ratio). At 48 h postinfection, cell-free supernatants were collected for the measurement of cytokine profiles via protein cytokine arrays. The intensities of protein spots for the IP-10-treated group were compared with those of the corresponding spots for the untreated controls, and data are presented as x-fold increases above the infection control levels. The data shown are the results for those molecules that displayed ≥2-fold increases over the infection control levels and are representative of three independent repeats.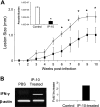
 , P < 0.05 (treated groups in comparison to the PBS controls). (B) At 10 weeks postinfection, PBS- and IP-10-treated mice were sacrificed, and total RNAs were extracted from infected foot tissues. RT-PCR analysis was performed to determine expression changes in IFN-γ. Band intensities were analyzed via spot densitometry, and expression changes were determined after normalization with β-actin.
, P < 0.05 (treated groups in comparison to the PBS controls). (B) At 10 weeks postinfection, PBS- and IP-10-treated mice were sacrificed, and total RNAs were extracted from infected foot tissues. RT-PCR analysis was performed to determine expression changes in IFN-γ. Band intensities were analyzed via spot densitometry, and expression changes were determined after normalization with β-actin.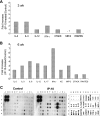
Similar articles
-
Effects of CXCL10 on dendritic cell and CD4+ T-cell functions during Leishmania amazonensis infection.Infect Immun. 2008 Jan;76(1):161-9. doi: 10.1128/IAI.00825-07. Epub 2007 Nov 12. Infect Immun. 2008. PMID: 17998308 Free PMC article.
-
Skin-derived macrophages from Leishmania major-susceptible mice exhibit interleukin-12- and interferon-gamma-independent nitric oxide production and parasite killing after treatment with immunostimulatory DNA.J Invest Dermatol. 2002 Sep;119(3):621-8. doi: 10.1046/j.1523-1747.2002.01850.x. J Invest Dermatol. 2002. PMID: 12230504
-
CD4+ Th1 cells induced by dendritic cell-based immunotherapy in mice chronically infected with Leishmania amazonensis do not promote healing.Infect Immun. 2004 Aug;72(8):4455-63. doi: 10.1128/IAI.72.8.4455-4463.2004. Infect Immun. 2004. PMID: 15271903 Free PMC article.
-
Cytokines and nitric oxide as effector molecules against parasitic infections.Philos Trans R Soc Lond B Biol Sci. 1997 Sep 29;352(1359):1311-5. doi: 10.1098/rstb.1997.0115. Philos Trans R Soc Lond B Biol Sci. 1997. PMID: 9355122 Free PMC article. Review.
-
Kinetoplastid Membrane Protein-11 as a Vaccine Candidate and a Virulence Factor in Leishmania.Front Immunol. 2015 Oct 13;6:524. doi: 10.3389/fimmu.2015.00524. eCollection 2015. Front Immunol. 2015. PMID: 26528287 Free PMC article. Review.
Cited by
-
Evaluation of Proinflammatory Chemokines in HIV Patients with Asymptomatic Leishmania Infantum Infection.Trop Med Infect Dis. 2023 Nov 9;8(11):495. doi: 10.3390/tropicalmed8110495. Trop Med Infect Dis. 2023. PMID: 37999614 Free PMC article.
-
Effects of CXCL10 on dendritic cell and CD4+ T-cell functions during Leishmania amazonensis infection.Infect Immun. 2008 Jan;76(1):161-9. doi: 10.1128/IAI.00825-07. Epub 2007 Nov 12. Infect Immun. 2008. PMID: 17998308 Free PMC article.
-
CXCL10 production by human monocytes in response to Leishmania braziliensis infection.Infect Immun. 2010 Jan;78(1):301-8. doi: 10.1128/IAI.00959-09. Epub 2009 Nov 9. Infect Immun. 2010. PMID: 19901067 Free PMC article.
-
Interferon gamma in leishmaniasis.Front Immunol. 2013 Jun 19;4:156. doi: 10.3389/fimmu.2013.00156. eCollection 2013. Front Immunol. 2013. PMID: 23801993 Free PMC article.
-
Deriving a Boolean dynamics to reveal macrophage activation with in vitro temporal cytokine expression profiles.BMC Bioinformatics. 2019 Dec 18;20(1):725. doi: 10.1186/s12859-019-3304-5. BMC Bioinformatics. 2019. PMID: 31852428 Free PMC article.
References
-
- Belkaid, Y., K. F. Hoffmann, S. Mendez, S. Kamhawi, M. C. Udey, T. A. Wynn, and D. L. Sacks. 2001. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J. Exp. Med. 194:1497-1506. - PMC - PubMed
-
- Bhattacharyya, S., S. Ghosh, B. Dasgupta, D. Mazumder, S. Roy, and S. Majumdar. 2002. Chemokine-induced leishmanicidal activity in murine macrophages via the generation of nitric oxide. J. Infect. Dis. 185:1704-1708. - PubMed
-
- Brandonisio, O., M. A. Panaro, I. Fumarola, M. Sisto, D. Leogrande, A. Acquafredda, R. Spinelli, and V. Mitolo. 2002. Macrophage chemotactic protein-1 and macrophage inflammatory protein-1alpha induce nitric oxide release and enhance parasite killing in Leishmania infantum-infected human macrophages. Clin. Exp. Med. 2:125-129. - PubMed
-
- Chen, J., B. P. Vistica, H. Takase, D. I. Ham, R. N. Fariss, E. F. Wawrousek, C. C. Chan, J. A. DeMartino, J. M. Farber, and I. Gery. 2004. A unique pattern of up- and down-regulation of chemokine receptor CXCR3 on inflammation-inducing Th1 cells. Eur. J. Immunol. 34:2885-2894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

