Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo
- PMID: 16982872
- PMCID: PMC6108417
- DOI: 10.4049/jimmunol.177.7.4376
Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo
Abstract
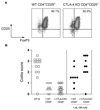
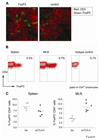
Naturally occurring CD4+ regulatory T cells (T(R)) that express CD25 and the transcription factor FoxP3 play a key role in immune homeostasis, preventing immune pathological responses to self and foreign Ags. CTLA-4 is expressed by a high percentage of these cells, and is often considered as a marker for T(R) in experimental and clinical analysis. However, it has not yet been proven that CTLA-4 has a direct role in T(R) function. In this study, using a T cell-mediated colitis model, we demonstrate that anti-CTLA-4 mAb treatment inhibits T(R) function in vivo via direct effects on CTLA-4-expressing T(R), and not via hyperactivation of colitogenic effector T cells. Although anti-CTLA-4 mAb treatment completely inhibits T(R) function, it does not reduce T(R) numbers or their homing to the GALT, suggesting the Ab mediates its function by blockade of a signal required for T(R) activity. In contrast to the striking effect of the Ab, CTLA-4-deficient mice can produce functional T(R), suggesting that under some circumstances other immune regulatory mechanisms, including the production of IL-10, are able to compensate for the loss of the CTLA-4-mediated pathway. This study provides direct evidence that CTLA-4 has a specific, nonredundant role in the function of normal T(R). This role has to be taken into account when targeting CTLA-4 for therapeutic purposes, as such a strategy will not only boost effector T cell responses, but might also break T(R)-mediated self-tolerance.
Figures


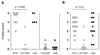


References
-
- Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389–400. - PubMed
-
- Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. - PubMed
-
- Pirzer U, Schonhaar A, Fleischer B, Hermann E, Meyer zum Buschenfelde KH. Reactivity of infiltrating T lymphocytes with microbial antigens in Crohn’s disease. Lancet. 1991;338:1238–1239. - PubMed
-
- Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

