Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention
- PMID: 16982929
- PMCID: PMC2855298
- DOI: 10.4049/jimmunol.177.7.4870
Novel approach to inhibit asthma-mediated lung inflammation using anti-CD147 intervention
Abstract
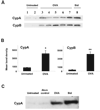
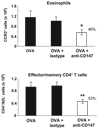
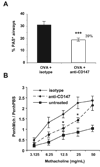
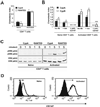
Extracellular cyclophilins have been well described as chemotactic factors for various leukocyte subsets. This chemotactic capacity is dependent upon interaction of cyclophilins with the cell surface signaling receptor CD147. Elevated levels of extracellular cyclophilins have been documented in several inflammatory diseases. We propose that extracellular cyclophilins, via interaction with CD147, may contribute to the recruitment of leukocytes from the periphery into tissues during inflammatory responses. In this study, we examined whether extracellular cyclophilin-CD147 interactions might influence leukocyte recruitment in the inflammatory disease allergic asthma. Using a mouse model of asthmatic inflammation, we show that 1) extracellular cyclophilins are elevated in the airways of asthmatic mice; 2) mouse eosinophils and CD4+ T cells express CD147, which is up-regulated on CD4+ T cells upon activation; 3) cyclophilins induce CD147-dependent chemotaxis of activated CD4+ T cells in vitro; 4) in vivo treatment with anti-CD147 mAb significantly reduces (by up to 50%) the accumulation of eosinophils and effector/memory CD4+ T lymphocytes, as well as Ag-specific Th2 cytokine secretion, in lung tissues; and 5) anti-CD147 treatment significantly reduces airway epithelial mucin production and bronchial hyperreactivity to methacholine challenge. These findings provide a novel mechanism whereby asthmatic lung inflammation may be reduced by targeting cyclophilin-CD147 interactions.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures




References
-
- Cohn L, Elias J, Chupp G. Asthma: mechanisms of disease persistence and progression. Annu. Rev. Immunol. 2004;22:789–815. - PubMed
-
- Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2003;111:450–463. - PubMed
-
- Bisset LR, Schmid-Grendelmeier P. Chemokines and their receptors in the pathogenesis of allergic asthma: progress and perspective. Curr. Opin. Pulm. Med. 2005;11:35–42. - PubMed
-
- Elsner J, Escher SE, Forssmann U. Chemokine receptor antagonists: a novel therapeutic approach in allergic diseases. Allergy. 2004;59:1243–1258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

