An alpha2-macroglobulin-like protein is the cue to gregarious settlement of the barnacle Balanus amphitrite
- PMID: 16983086
- PMCID: PMC1599974
- DOI: 10.1073/pnas.0602763103
An alpha2-macroglobulin-like protein is the cue to gregarious settlement of the barnacle Balanus amphitrite
Abstract
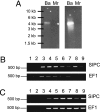
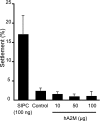
Many benthic marine invertebrates, like barnacles, have a planktonic larval stage whose primary purpose is dispersal. How these species colonize suitable substrata is fundamental to understanding their evolution, population biology, and wider community dynamics. Unlike larval dispersal, settlement occurs on a relatively small spatial scale and involves larval behavior in response to physical and chemical characteristics of the substratum. Biogenic chemical cues have been implicated in this process. Their identification, however, has proven challenging, no more so than for the chemical basis of barnacle gregariousness, which was first described >50 years ago. We now report that a biological cue to gregarious settlement, the settlement-inducing protein complex (SIPC), of the major fouling barnacle Balanus amphitrite is a previously undescribed glycoprotein. The SIPC shares a 30% sequence homology with the thioester-containing family of proteins that includes the alpha(2)-macroglobulins. The cDNA (5.2 kb) of the SIPC encodes a protein precursor comprising 1,547 aa with a 17-residue signal peptide region. A number of structural characteristics and the absence of a thioester bond in the SIPC suggest that this molecule is a previously undescribed protein that may have evolved by duplication from an ancestral alpha(2)-macroglobulin gene. Although the SIPC is regarded as an adult cue that is recognized by the cyprid at settlement, it is also expressed in the juvenile and in larvae, where it may function in larva-larva settlement interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Darwin C. A Monograph of the Sub-class Cirripedia, with Figures of all the Species. The Balanidae (or Sessile Cirripedes); the Verrucidae. London: Ray Society; 1854.
-
- Matthiessen P, Gibbs PE. Environ Toxicol Chem. 1998;17:37–43.
-
- Steinberg PD, de Nys R, Kjelleberg S. In: Marine Chemical Ecology. McClintock JB, Baker JB, editors. Boca Raton, FL: CRC; 2001. pp. 355–387.
-
- Knight-Jones EW, Crisp DJ. Nature. 1953;171:1109–1110. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

