Viral oncoproteins target the DNA methyltransferases
- PMID: 16983344
- PMCID: PMC3350866
- DOI: 10.1038/sj.onc.1209950
Viral oncoproteins target the DNA methyltransferases
Abstract
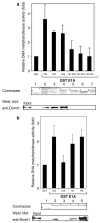
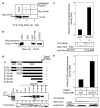
Small DNA tumour viruses have evolved a number of mechanisms to drive nondividing cells into S phase. Virally encoded oncoproteins such as adenovirus E1A and human papillomavirus (HPV) E7 can bind an array of cellular proteins to override proliferation arrest. The DNA methyltransferase Dnmt1 is the major mammalian enzyme responsible for maintaining CpG methylation patterns in the cell following replication. One of the hallmarks of tumour cells is disrupted DNA methylation patterns, highlighting the importance of the proper regulation of DNA methyltransferases in normal cell proliferation. Here, we show that adenovirus 5 E1A and HPV-16 E7 associate in vitro and in vivo with the DNA methyltransferase Dnmt1. Consistent with this interaction, we find that E1A and E7 can purify DNA methyltransferase activity from nuclear extracts. These associations are direct and mediated by the extreme N-terminus of E1A and the CR3 zinc-finger domain of E7. Furthermore, we find that a point mutant at leucine 20 of E1A, a residue known to be critical for its transformation functions, is unable to bind Dnmt1 and DNA methyltransferase activity. Finally, both E1A and E7 can stimulate the methyltransferase activity of Dnmt1 in vitro. Our results provide the first indication that viral oncoproteins bind and regulate Dnmt1 enzymatic activity. These observations open up the possibility that this association may be used to control cellular proliferation pathways and suggest a new mechanism by which small DNA tumour viruses can steer cells through the cell cycle.
Figures


References
-
- Ait-Si-Ali S, Ramirez S, Barre FX, Dkhissi F, Magnaghi-Jaulin L, Girault JA, et al. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature. 1998;396:184–186. - PubMed
-
- Chakravarti D, Ogryzko V, Kao HY, Nash A, Chen H, Nakatani Y, et al. A viral mechanism for inhibition of p300 and PCAF acetyltransferase activity. Cell. 1999;96:393–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

