Stratus not altocumulus: a new view of the yeast protein interaction network
- PMID: 16984220
- PMCID: PMC1569888
- DOI: 10.1371/journal.pbio.0040317
Stratus not altocumulus: a new view of the yeast protein interaction network
Abstract
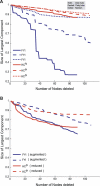
Systems biology approaches can reveal intermediary levels of organization between genotype and phenotype that often underlie biological phenomena such as polygenic effects and protein dispensability. An important conceptualization is the module, which is loosely defined as a cohort of proteins that perform a dedicated cellular task. Based on a computational analysis of limited interaction datasets in the budding yeast Saccharomyces cerevisiae, it has been suggested that the global protein interaction network is segregated such that highly connected proteins, called hubs, tend not to link to each other. Moreover, it has been suggested that hubs fall into two distinct classes: "party" hubs are co-expressed and co-localized with their partners, whereas "date" hubs interact with incoherently expressed and diversely localized partners, and thereby cohere disparate parts of the global network. This structure may be compared with altocumulus clouds, i.e., cotton ball-like structures sparsely connected by thin wisps. However, this organization might reflect a small and/or biased sample set of interactions. In a multi-validated high-confidence (HC) interaction network, assembled from all extant S. cerevisiae interaction data, including recently available proteome-wide interaction data and a large set of reliable literature-derived interactions, we find that hub-hub interactions are not suppressed. In fact, the number of interactions a hub has with other hubs is a good predictor of whether a hub protein is essential or not. We find that date hubs are neither required for network tolerance to node deletion, nor do date hubs have distinct biological attributes compared to other hubs. Date and party hubs do not, for example, evolve at different rates. Our analysis suggests that the organization of global protein interaction network is highly interconnected and hence interdependent, more like the continuous dense aggregations of stratus clouds than the segregated configuration of altocumulus clouds. If the network is configured in a stratus format, cross-talk between proteins is potentially a major source of noise. In turn, control of the activity of the most highly connected proteins may be vital. Indeed, we find that a fluctuation in steady-state levels of the most connected proteins is minimized.
Conflict of interest statement
Competing interests. The authors have declared that no competing interests exist.
Figures





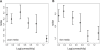
Comment in
-
Confirmation of organized modularity in the yeast interactome.PLoS Biol. 2007 Jun;5(6):e153. doi: 10.1371/journal.pbio.0050153. PLoS Biol. 2007. PMID: 17564493 Free PMC article. No abstract available.
-
For yeast protein hubs, more data means more connections.PLoS Biol. 2006 Oct;4(10):e331. doi: 10.1371/journal.pbio.0040331. Epub 2006 Sep 19. PLoS Biol. 2006. PMID: 20076470 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

