Helicobacter pylori infection stimulates intestinalization of endocrine cells in glandular stomach of Mongolian gerbils
- PMID: 16984375
- PMCID: PMC11158682
- DOI: 10.1111/j.1349-7006.2006.00273.x
Helicobacter pylori infection stimulates intestinalization of endocrine cells in glandular stomach of Mongolian gerbils
Abstract
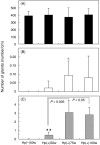
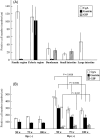
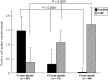
Intestinal metaplasia has been investigated extensively as a possible premalignant condition for stomach cancer but its pathogenesis is still not fully understood. In the present study, we examined the relationship between endocrine and mucous cell marker expression periodically after Helicobacter pylori infection in the Mongolian gerbil model. The numbers of chromogranin A (CgA)-positive, gastrin-positive and gastric inhibitory polypeptide (GIP)-positive cells in H. pylori-infected groups was increased significantly compared with the non-infected case. However, CgA-positive and gastrin-positive cells then decreased from 50 through 100 experimental weeks after H. pylori infection, whereas GIP-positive cells increased. Coexistence of gastrin-positive and GIP-positive cells was detected in the same gastric and intestinal mixed phenotypic glandular-type glands. In conclusion, the endocrine cell phenotype is in line with that of the mucous counterpart in the glands of H. pylori-infected Mongolian gerbil stomach, supporting the concept that development of intestinal metaplasia is due to the abnormal differentiation of a stem cell.
Figures




Similar articles
-
Coexistence of gastric- and intestinal-type endocrine cells in gastric and intestinal mixed intestinal metaplasia of the human stomach.Pathol Int. 2005 Apr;55(4):170-9. doi: 10.1111/j.1440-1827.2005.01809.x. Pathol Int. 2005. PMID: 15826243
-
Gastric and intestinal phenotypes and histogenesis of advanced glandular stomach cancers in carcinogen-treated, Helicobacter pylori-infected Mongolian gerbils.Cancer Sci. 2006 Jan;97(1):38-44. doi: 10.1111/j.1349-7006.2006.00135.x. Cancer Sci. 2006. PMID: 16367919 Free PMC article.
-
Helicobacter pylori infection and high dietary salt independently induce atrophic gastritis and intestinal metaplasia in commercially available outbred Mongolian gerbils.Dig Dis Sci. 2003 Mar;48(3):475-85. doi: 10.1023/a:1022524313355. Dig Dis Sci. 2003. PMID: 12757158
-
Role of Helicobacter pylori in gastric carcinogenesis: the origin of gastric cancers and heterotopic proliferative glands in Mongolian gerbils.Helicobacter. 2005 Apr;10(2):97-106. doi: 10.1111/j.1523-5378.2005.00305.x. Helicobacter. 2005. PMID: 15810939 Review.
-
Metaplasia in the Stomach-Precursor of Gastric Cancer?Int J Mol Sci. 2017 Sep 27;18(10):2063. doi: 10.3390/ijms18102063. Int J Mol Sci. 2017. PMID: 28953255 Free PMC article. Review.
Cited by
-
In vitro differentiation of Runx3-/- p53-/- gastric epithelial cells into intestinal type cells.Cancer Sci. 2008 Apr;99(4):671-6. doi: 10.1111/j.1349-7006.2008.00732.x. Cancer Sci. 2008. PMID: 18377419 Free PMC article.
-
Diversity in bacterium-host interactions within the species Helicobacter heilmannii sensu stricto.Vet Res. 2013 Jul 29;44(1):65. doi: 10.1186/1297-9716-44-65. Vet Res. 2013. PMID: 23895283 Free PMC article.
-
Animal models to study the role of long-term hypergastrinemia in gastric carcinogenesis.J Biomed Biotechnol. 2011;2011:975479. doi: 10.1155/2011/975479. Epub 2010 Nov 24. J Biomed Biotechnol. 2011. PMID: 21127707 Free PMC article. Review.
References
-
- Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol 1996; 31 (Suppl. 9): 24–8. - PubMed
-
- Wong BC, Lam SK, Wong WM et al. Helicobacter pylori eradication to prevent gastric cancer in a high‐risk region of China: a randomized controlled trial. JAMA 2004; 291: 187–94. - PubMed
-
- Mingazzini P, Carlei F, Malchiodi‐Albedi F et al. Endocrine cells in intestinal metaplasia of the stomach. J Pathol 1984; 144: 171–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

