Regulation of cytoskeletal dynamics at the immune synapse: new stars join the actin troupe
- PMID: 16984404
- PMCID: PMC1779662
- DOI: 10.1111/j.1600-0854.2006.00491.x
Regulation of cytoskeletal dynamics at the immune synapse: new stars join the actin troupe
Abstract
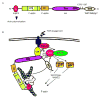
Reorganization of actin cytoskeletal dynamics plays a critical role in controlling T-lymphocyte activation and effector functions. Interaction of T-cell receptors (TCR) with appropriate major histocompatibility complex-peptide complexes on antigen-presenting cells results in the activation of signaling cascades, leading to the accumulation of F-actin at the cell-cell contact site. This event is required for the formation and stabilization of the immune synapse (IS), a cellular structure essential for the modulation of T-cell responses. Analysis of actin cytoskeletal dynamics following engagement of the TCR has largely focused on the Arp2/3 regulator, WASp, because of its early identification and its association with human disease. However, recent studies have shown equally important roles for several additional actin regulatory proteins. In this review, we turn the spotlight on the expanding cast of actin regulatory proteins, which co-ordinate actin dynamics at the IS.
Figures



References
-
- Ryser JE, Rungger-Brandle E, Chaponnier C, Gabbiani G, Vassalli P. The area of attachment of cytotoxic T lymphocytes to their target cells shows high motility and polarization of actin, but not myosin. J Immunol. 1982;128:1159–1162. - PubMed
-
- Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23–29. - PubMed
-
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. - PubMed
-
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

