Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children's Oncology Group
- PMID: 16985182
- PMCID: PMC1785079
- DOI: 10.1182/blood-2006-01-023101
Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children's Oncology Group
Abstract
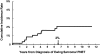
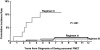
This study describes the magnitude of risk of therapy-related myelodysplasia and acute myeloid leukemia (t-MDS/AML) in 578 individuals diagnosed with Ewing sarcoma and enrolled on Children's Oncology Group therapeutic protocol, INT-0091. Between 1988 and 1992, patients with or without metastatic disease were randomized to receive doxorubicin, vincristine, cyclophosphamide, and dactinomycin (regimen A) or these 4 drugs alternating with etoposide and ifosfamide (regimen B). Between 1992 and 1994, patients with metastatic disease were nonrandomly assigned to receive high-intensity therapy (regimen C: regimen B therapy with higher doses of doxorubicin, cyclophosphamide, and ifosfamide). Median age at diagnosis of Ewing sarcoma was 12 years, and median length of follow-up, 8 years. Eleven patients developed t-MDS/AML, resulting in a cumulative incidence of 2% at 5 years. While patients treated on regimens A and B were at a low risk for development of t-MDS/AML (cumulative incidence: 0.4% and 0.9% at 5 years, respectively), patients treated on regimen C were at a 16-fold increased risk of developing t-MDS/AML (cumulative incidence: 11% at 5 years), when compared with those treated on regimen A. Increasing exposure to ifosfamide from 90 to 140 g/m2, cyclophosphamide from 9.6 to 17.6 g/m2, and doxorubicin from 375 to 450 mg/m2 increased the risk of t-MDS/AML significantly.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Nesbit ME. Ewing's sarcoma. CA Cancer J Clin. 1976;26:174–180. - PubMed
-
- Hustu HO, Pinkel D, Pratt CB. Treatment of clinically localized Ewing's sarcoma with radiotherapy and combination chemotherapy. Cancer. 1972;30:1522–1527. - PubMed
-
- Jurgens H, Exner U, Kuhl J, et al. High-dose ifosfamide with mesna uroprotection in Ewing's sarcoma. Cancer Chemother Pharmacol. 1989;24(suppl 1):S40–S44. - PubMed
-
- Jurgens H, Exner U, Gadner H, et al. Multidisciplinary treatment of primary Ewing's sarcoma of bone: a 6-year experience of a European Cooperative Trial. Cancer. 1988;61:23–32. - PubMed
-
- Nesbit MEJ, Gehan EA, Burgert EOJ, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: a long-term follow-up of the First Intergroup study. J Clin Oncol. 1990;8:1664–1674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

