Comparison of clinical trials with finasteride and dutasteride
- PMID: 16985923
- PMCID: PMC1472914
Comparison of clinical trials with finasteride and dutasteride
Abstract
Finasteride selectively inhibits the Type 2 isoenzyme of 5alpha-reductase (5AR) (the enzyme responsible for converting testosterone to dihydrotestosterone [DHT]) whereas dutasteride inhibits both Type 1 and Type 2 5AR. General conclusions regarding the differences and similarities of these 2 agents, in terms of pharmacologic effect, safety, and efficacy, can be drawn from evaluation of short-term comparative trials and similar but non-comparative long-term trials. Dutasteride therapy reduces serum DHT significantly more than does finasteride. In men with benign prostatic hyperplasia (BPH), treatment with either agent results in similar prostate gland volume reduction, flow rate and symptom improvement, and similar reductions in long-term risk of BPH development in terms of symptom progression and acute urinary retention (AUR) and BPH-related surgery. There does not appear to be any clinically significant difference between the adverse event profiles of dutasteride and finasteride. Although weak evidence suggests a difference in the onset of clinical benefit, the available non-comparative trial data do not confirm this finding. Patients with symptomatic BPH who receive dutasteride or finasteride, either as monotherapy or combination therapy with alpha-blockers, can expect to experience significant prostate gland size reduction, improved symptoms, and reduced risk of progression in terms of long-term adverse outcomes.
Figures

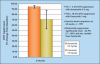
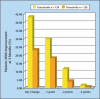


References
-
- Bartsch G, Rittmaster RS, Klocker H. Dihydrotestosterone and the concept of 5α-reductase inhibition in human benign prostatic hyperplasia. Eur Urol. 2000;37:367–380. - PubMed
-
- Steers WD. 5α-reductase activity in the prostate. Urol. 2001;58(suppl 6A):17–24. - PubMed
-
- Clark RV, Hermann DJ, Cunningham GR, et al. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5α-Reductase inhibitor. J Clin Endocrin Metab. 2004;89:2179–2184. - PubMed
-
- Roehrborn CG, Boyle P, Nickel JC, et al. Efficacy and safety of a dual inhibitor of 5α-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urol. 2002;60:434–441. - PubMed
-
- McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Engl J Med. 1998;338:557–563. - PubMed
LinkOut - more resources
Full Text Sources
