5-alpha-Reductase Inhibitors Prevent the Progression of Benign Prostatic Hyperplasia
- PMID: 16985965
- PMCID: PMC1502358
5-alpha-Reductase Inhibitors Prevent the Progression of Benign Prostatic Hyperplasia
Abstract
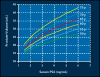
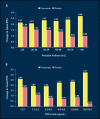
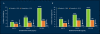
Lower urinary tract symptoms (LUTS) associated with clinical benign prostatic hyperplasia (BPH) are a common occurrence in aging men, causing bother and interference with daily activities and affecting disease-specific quality of life. There is increasing evidence to suggest that, in many patients, the signs and symptoms of BPH are progressive. Progression can be measured as continued growth of the prostate gland; worsening of symptoms, bother, or quality of life; deterioration of urinary flow rate; episodes of acute urinary retention (AUR); and need for prostate-related surgery. Furthermore, it has become clear that the risk of disease progression increases with age as well as with increasing prostate volume and serum prostate-specific antigen (PSA) level. The 5-alpha-reductase inhibitor finasteride has been shown not only to improve symptoms, bother, and quality of life but also to prevent progression to AUR and surgery, with a relative risk reduction of over 50%. As the risk for such progression is higher in patients with larger glands or higher serum PSA values at baseline, it is in those patients that finasteride induces an even greater risk reduction, making it a cost-effective treatment choice for patients with LUTS associated with prostatic enlargement.
Figures




References
-
- Girman CJ, Jacobsen SJ, Tsukamoto T, et al. Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology. 1998;51:428–436. - PubMed
-
- Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469–471. - PubMed
-
- Jacobsen SJ, Girman CJ, Guess HA, et al. New diagnostic and treatment guidelines for benign prostatic hyperplasia: potential impact in the United States. Arch Intern Med. 1995;155:477–481. - PubMed
-
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. - PubMed
-
- McConnell JD. Benign prostatic hyperplasia: hormonal treatment. Urol Clin North Am. 1995;22:387–400. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
