Flare Associated with LHRH-Agonist Therapy
Abstract
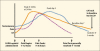
The most common form of hormonal treatment for prostate cancer is luteinizing hormone-releasing hormone (LHRH)-agonist therapy. During the first 1 to 3 weeks of LHRH-agonist therapy, an initial increase in testosterone is associated with a condition known as "flare." Blockade of flare can be accomplished with a number of agents, including flutamide, bicalutamide, nilutamide, diethylstilbestrol, ketoconazole, and cyproterone acetate. Evidence from the early use of LHRHagonists suggested that flare could be serious in nature, with an exacerbation of pain, increase in uremia, development of neurologic sequelae, and possibly death. These events have been uncommonly reported of late, most probably owing to the use of flare blockade in most patients with advanced disease, as well as the fact that many patients are currently being treated with much earlier disease. Evidence is conflicting as to whether flare makes a difference in less advanced disease. A few reports have noted complications during flare in patients in whom a blockade of flare was not required, including two deaths from one institution. Reported series, however, seem to suggest that with flare blockade, acute complications are extremely uncommon. Evidence suggests that 1) advanced disease should be blocked; 2) blockade should probably include an antiandrogen beginning about 1 week prior to administration of the LHRH-agonist; and 3) that patients without advanced disease but with very high PSA levels should be considered for flare blockade.
Figures
References
-
- Agency for Health Care Policy and Research, authors. Relative Effectiveness and Cost-Effectiveness of Methods of Androgen Suppression in the Treatment of Advanced Prostatic Cancer. U.S. Department of Health and Human Services. 1999. May, (AHCPR Publication No. 99-E0012).
-
- Kreis W, Ahmann FR, Jordan VC, et al. Oestrogen pre-treatment abolishes luteinising hormone-releasing hormone testosterone stimulation. Br J Urol. 1988;62:352–354. - PubMed
-
- Boccon-Gibod L, Laudat MH, Dugue MA, Steg A. Cyproterone acetate lead-in prevents initial rise of serum testosterone induced by luteinizing hormone-releasing hormone analogs in the treatment of metastatic carcinoma of the prostate. Eur Urol. 1986;12:400–402. - PubMed
-
- Schroeder FH, Lock TMTW, Chadha DR, et al. Metastatic cancer of the prostate managed with buserelin versus buserelin plus cyproterone acetate. J Urol. 1987;137:912–918. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous