Pathophysiology of overactive bladder and urge urinary incontinence
- PMID: 16986023
- PMCID: PMC1476015
Pathophysiology of overactive bladder and urge urinary incontinence
Abstract
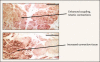
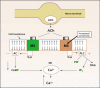
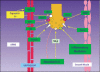
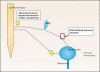
Storage symptoms such as urgency, frequency, and nocturia, with or without urge incontinence, are characterized as overactive bladder (OAB). OAB can lead to urge incontinence. Disturbances in nerves, smooth muscle, and urothelium can cause this condition. In some respects the division between peripheral and central causes of OAB is artificial, but it remains a useful paradigm for appreciating the interactions between different tissues. Models have been developed to mimic the OAB associated with bladder instability, lower urinary tract obstruction, neuropathic disorders, diabetes, and interstitial cystitis. These models share the common features of increased connectivity and excitability of both detrusor smooth muscle and nerves. Increased excitability and connectivity of nerves involved in micturition rely on growth factors that orchestrate neural plasticity. Neurotransmitters, prostaglandins, and growth factors, such as nerve growth factor, provide mechanisms for bidirectional communication between muscle or urothelium and nerve, leading to OAB with or without urge incontinence.
Figures

References
-
- Eckhardt MD, van Venrooij GE, Boon TA. Symptoms, prostate volume, and urodynamic findings in elderly male volunteers without and with LUTS and in patients with LUTS suggestive of benign prostatic hyperplasia. Urology. 2001;58:966–971. - PubMed
-
- Sibley GNA. The Response of the Bladder to Lower Urinary Tract Obstruction [DM thesis] Oxford, England: Oxford University; 1984.
-
- German K, Bedwani J, Davies J, et al. What is the pathophysiology of detrusor hyperreflexia? Neurourol Urodyn. 1993;12:335–336.
-
- Sibley GNA. Developments in our understanding of detrusor instability. Br J Urol. 1997;80:54–61. - PubMed
-
- Brading AF, Turner WH. The unstable bladder: towards a common mechanism. Br J Urol. 1994;73:3–8. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
