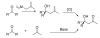Catalytic asymmetric methallylation of ketones with an (H8-BINOLate)Ti-based catalyst
- PMID: 16986913
- PMCID: PMC2615050
- DOI: 10.1021/ol061417y
Catalytic asymmetric methallylation of ketones with an (H8-BINOLate)Ti-based catalyst
Abstract
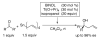
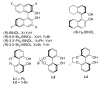
The first catalytic asymmetric methallylation of ketones is reported. The catalyst, which is generated from titanium tetraisopropoxide, H8-BINOL, 2-propanol, and tetramethallylstannane, reacts with ketones in acetonitrile to afford tertiary homoallylic alcohols in fair to excellent yields (55-99%) and fair to high enantioselectivities (46-90%). Ozonolysis of the resulting products provides access to chiral beta-hydroxy ketones, which are not readily prepared from direct asymmetric aldol reaction of acetone with ketones.
Figures
References
-
- Yanagisawa A. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 2. Springer; Berlin: 1999. pp. 965–979.
-
- Pu L. Tetrahedron. 2003;59:9873–9886.
-
- Pu L, Yu H-B. Chem. Rev. 2001;101:757–824. - PubMed
-
- Soai K, Niwa S. Chem. Rev. 1992;92:833–856.
-
- Denmark SE, Fu J. Chem. Rev. 2003;103:2763–2794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources