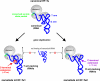
Intricacies and surprises of nuclear-mitochondrial co-evolution
- PMID: 16987107
- PMCID: PMC1609910
- DOI: 10.1042/BJ20061241
Intricacies and surprises of nuclear-mitochondrial co-evolution
Abstract
In this issue of the Biochemical Journal, Watanabe and colleagues disclose another fascinating facet of the mitochondrial protein synthesis machinery: one of the two nematode mitochondrial elongation factors Tu, EF-Tu1, specifically recognizes the D-arm of T-armless tRNAs via a 57-amino-acid C-terminal extension that compensates for the reduction in tRNA structure. This principle provides a paradigm for the evolutionary events thought to have ignited the transition from an ancient 'RNA world' to the 'protein world' of today.
Figures
Comment on
-
A protein extension to shorten RNA: elongated elongation factor-Tu recognizes the D-arm of T-armless tRNAs in nematode mitochondria.Biochem J. 2006 Oct 15;399(2):249-56. doi: 10.1042/BJ20060781. Biochem J. 2006. PMID: 16859488 Free PMC article.
References
-
- Ohtsuki T., Sato A., Watanabe Y., Watanabe K. A unique serine-specific elongation factor Tu found in nematode mitochondria. Nat. Struct. Biol. 2002;9:669–673. - PubMed
-
- Erratum. Nat. Struct. Biol. 2003;10:669.
-
- Cousineau B., Leclerc F., Cedergren R. On the origin of protein synthesis factors: a gene duplication/fusion model. J. Mol. Evol. 1997;45:661–670. - PubMed
-
- Wilson D. N., Nierhaus K. H. The ribosome through the looking glass. Angew. Chem. Int. Ed. Engl. 2003;42:3464–3486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources