The natural compound n-butylidenephthalide derived from Angelica sinensis inhibits malignant brain tumor growth in vitro and in vivo
- PMID: 16987298
- PMCID: PMC1804119
- DOI: 10.1111/j.1471-4159.2006.04151.x
The natural compound n-butylidenephthalide derived from Angelica sinensis inhibits malignant brain tumor growth in vitro and in vivo
Abstract
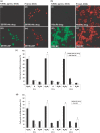
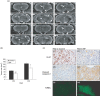
The naturally-occurring compound, n-butylidenephthalide (BP), which is isolated from the chloroform extract of Angelica sinensis (AS-C), has been investigated with respect to the treatment of angina. In this study, we have examined the anti-tumor effects of n-butylidenephthalide on glioblastoma multiforme (GBM) brain tumors both in vitro and in vivo. In vitro, GBM cells were treated with BP, and the effects of proliferation, cell cycle and apoptosis were determined. In vivo, DBTRG-05MG, the human GBM tumor, and RG2, the rat GBM tumor, were injected subcutaneously or intracerebrally with BP. The effects on tumor growth were determined by tumor volumes, magnetic resonance imaging and survival rate. Here, we report on the potency of BP in suppressing growth of malignant brain tumor cells without simultaneous fibroblast cytotocixity. BP up-regulated the expression of Cyclin Kinase Inhibitor (CKI), including p21 and p27, to decrease phosphorylation of Rb proteins, and down-regulated the cell-cycle regulators, resulting in cell arrest at the G(0)/G(1) phase for DBTRG-05MG and RG2 cells, respectively. The apoptosis-associated proteins were dramatically increased and activated by BP in DBTRG-05MG cells and RG2 cells, but RG2 cells did not express p53 protein. In vitro results showed that BP triggered both p53-dependent and independent pathways for apoptosis. In vivo, BP not only suppressed growth of subcutaneous rat and human brain tumors but also, reduced the volume of GBM tumors in situ, significantly prolonging survival rate. These in vitro and in vivo anti-cancer effects indicate that BP could serve as a new anti-brain tumor drug.
Figures



References
-
- Ye Y N, Koo M W, Li Y, Matsui H, Cho C H. Angelica sinensis modulates migration and proliferation of gastric epithelial cells. Life Sci. 2001a;68:961–968. - PubMed
-
- Ye Y N, Liu E S, Li Y. Protective effect of polysaccharides-enriched fraction from Angelica sinensis on hepatic injury. Life Sci. 2001b;69:637–646. - PubMed
-
- Graham D I, Lantos P L. Greenfield's Neuropathology. 7. London: Hodder Arnold Press; 2002.
-
- Santarius T, Kirsch M, Rossi M L, Black P M. Molecular aspects of neuro-oncology. ClinNeurolNeurosurg. 1997;99:184–195. - PubMed
-
- Giese A, Bjerkvig R, Berens M E, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. JClinOncol. 2003;21:1624–1636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

