Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts
- PMID: 16987426
- PMCID: PMC1779441
- DOI: 10.1186/ar2046
Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts
Abstract
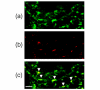
Increased bone resorption mediated by osteoclasts causes various diseases such as osteoporosis and bone erosion in rheumatoid arthritis (RA). Osteoclasts are derived from the monocyte/macrophage lineage, but the precise origin remains unclear. In the present study, we show that the purified CD16- human peripheral blood monocyte subset, but not the CD16+ monocyte subset, differentiates into osteoclast by stimulation with receptor activator of NF-kappaB ligand (RANKL) in combination with macrophage colony-stimulating factor (M-CSF). Integrin-beta3 mRNA and the integrin-alpha(v)beta3 heterodimer were only expressed on CD16- monocytes, when they were stimulated with RANKL + M-CSF. Downregulation of beta3-subunit expression by small interfering RNA targeting beta3 abrogated osteoclastogenesis from the CD16- monocyte subset. In contrast, the CD16+ monocyte subset expressed larger amounts of tumor necrosis factor alpha and IL-6 than the CD16- subset, which was further enhanced by RANKL stimulation. Examination of RA synovial tissue showed accumulation of both CD16+ and CD16- macrophages. Our results suggest that peripheral blood monocytes consist of two functionally heterogeneous subsets with distinct responses to RANKL. Osteoclasts seem to originate from CD16- monocytes, and integrin beta3 is necessary for osteoclastogenesis. Blockade of accumulation and activation of CD16- monocytes could therefore be a beneficial approach as an anti-bone resorptive therapy, especially for RA.
Figures


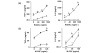




References
-
- Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

