Apoplastic pH during low-oxygen stress in Barley
- PMID: 16987922
- PMCID: PMC3292248
- DOI: 10.1093/aob/mcl193
Apoplastic pH during low-oxygen stress in Barley
Abstract
Background and aims: Anoxia leads to an energy crisis, tolerance of which varies from plant to plant. Although the apoplast represents an important storage and reaction space, and engages in the mediation of membrane transport, this extracellular compartment has not yet been granted a role during oxygen shortage. Here, an attempt is made to highlight the importance of the apoplast during oxygen stress and to test whether information about it is transferred systemically in Hordeum vulgare.
Methods: Non-invasive ion-selective microprobes were used which, after being inserted through open stomata, directly contact the apoplastic fluid and continuously measure the apoplastic pH and changes to it.
Key results: (a) Barley leaves respond to oxygen stress with apoplastic alkalinization and membrane depolarization. These responses are persistent under anoxia (N2; O2 < 3%) but transient under hypoxia. (b) Being applied to the root, the information 'anoxia' is signalled to the leaf as an increase in pH, whereas 'hypoxia' is not: flooding of the roots within the first 2 h has no effect on the leaf apoplastic pH, whereas anoxia (N2) or chemical anoxia (NaCN/salicylic hydroxamic acid) rapidly increase the leaf apoplastic pH. (c) Under anoxia, the proton motive force suffers a decrease by over 70 %, which impairs H(+) -driven transport.
Conclusions: Although anoxia-induced apoplastic alkalinization is a general response to stress, its impact on the proton motive force (reduction) and thus on transport mediation of energy-rich compounds is evident. It is concluded that anoxia tolerance depends on how the plant is able to hold the proton motive force and H(+) turnover at a level that guarantees sufficient energy is harvested to overcome the crisis.
Figures
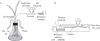

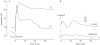



Similar articles
-
pH regulation in apoplastic and cytoplasmic cell compartments of leaves.Planta. 2000 Jul;211(2):246-55. doi: 10.1007/s004250000280. Planta. 2000. PMID: 10945219
-
Interactive signal transfer between host and pathogen during successful infection of barley leaves by Blumeria graminis and Bipolaris sorokiniana.J Plant Physiol. 2008 Jan;165(1):52-9. doi: 10.1016/j.jplph.2007.08.006. Epub 2007 Oct 1. J Plant Physiol. 2008. PMID: 17905475
-
Root-to-shoot signalling: apoplastic alkalinization, a general stress response and defence factor in barley (Hordeum vulgare).Protoplasma. 2005 Dec;227(1):17-24. doi: 10.1007/s00709-005-0131-5. Epub 2005 Dec 30. Protoplasma. 2005. PMID: 16389490
-
The pH of the Apoplast: Dynamic Factor with Functional Impact Under Stress.Mol Plant. 2017 Nov 6;10(11):1371-1386. doi: 10.1016/j.molp.2017.09.018. Epub 2017 Oct 5. Mol Plant. 2017. PMID: 28987886 Review.
-
pH regulation in anoxic plants.Ann Bot. 2005 Sep;96(4):519-32. doi: 10.1093/aob/mci207. Epub 2005 Jul 15. Ann Bot. 2005. PMID: 16024558 Free PMC article. Review.
Cited by
-
Boron Alleviates Aluminum Toxicity by Promoting Root Alkalization in Transition Zone via Polar Auxin Transport.Plant Physiol. 2018 Jul;177(3):1254-1266. doi: 10.1104/pp.18.00188. Epub 2018 May 21. Plant Physiol. 2018. PMID: 29784768 Free PMC article.
-
The infiltration-centrifugation technique for extraction of apoplastic fluid from plant leaves using Phaseolus vulgaris as an example.J Vis Exp. 2014 Dec 19;(94):52113. doi: 10.3791/52113. J Vis Exp. 2014. PMID: 25549068 Free PMC article.
-
System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding.Plant Physiol. 2009 Mar;149(3):1593-600. doi: 10.1104/pp.108.133884. Epub 2009 Jan 7. Plant Physiol. 2009. PMID: 19129416 Free PMC article.
-
Dissection of heat-induced systemic signals: superiority of ion fluxes to voltage changes in substomatal cavities.Planta. 2009 Feb;229(3):539-47. doi: 10.1007/s00425-008-0850-x. Epub 2008 Nov 15. Planta. 2009. PMID: 19011895
-
Comparative transcriptomic analysis of germinating rice seedlings to individual and combined anaerobic and cold stress.BMC Genomics. 2023 Apr 6;24(1):185. doi: 10.1186/s12864-023-09262-z. BMC Genomics. 2023. PMID: 37024819 Free PMC article.
References
-
- Fan TW-M, Higashi RM, Lane AN. (1988) An in vivo 1H and 31P NMR investigation of the effect of nitrate on hypoxic metabolism in maize roots. Archives of Biochemistry and Biophysics 266592–606. - PubMed
-
- Felle H. (1987) Proton transport and pH control in Sinapis root hairs: a study carried out with double-barrelled pH micro-electrodes. Journal of Experimental Botany 38340–354.
-
- Felle HH. (1996) Control of cytoplasmic pH under anoxic conditions and its implication for plasma membrane proton transport in Medicago sativa root hairs. Journal of Experimental Botany 47967–973.
-
- Felle H and Bertl A. (1986) The fabrication of H+-selective liquid-membrane micro-electrodes for use in plant cells. Journal of Experimental Botany 371416–1428.

