Myristoylated, alanine-rich C-kinase substrate phosphorylation regulates growth cone adhesion and pathfinding
- PMID: 16987960
- PMCID: PMC1679677
- DOI: 10.1091/mbc.e05-12-1183
Myristoylated, alanine-rich C-kinase substrate phosphorylation regulates growth cone adhesion and pathfinding
Abstract
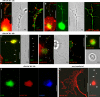
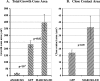
Repellents evoke growth cone turning by eliciting asymmetric, localized loss of actin cytoskeleton together with changes in substratum attachment. We have demonstrated that semaphorin-3A (Sema3A)-induced growth cone detachment and collapse require eicosanoid-mediated activation of protein kinase C epsilon (PKC epsilon) and that the major PKC epsilon target is the myristoylated, alanine-rich C-kinase substrate (MARCKS). Here, we show that PKC activation is necessary for growth cone turning and that MARCKS, while at the membrane, colocalizes with alpha3-integrin in a peripheral adhesive zone of the growth cone. Phosphorylation of MARCKS causes its translocation from the membrane to the cytosol. Silencing MARCKS expression dramatically reduces growth cone spread, whereas overexpression of wild-type MARCKS inhibits growth cone collapse triggered by PKC activation. Expression of phosphorylation-deficient, mutant MARCKS greatly expands growth cone adhesion, and this is characterized by extensive colocalization of MARCKS and alpha3-integrin, resistance to eicosanoid-triggered detachment and collapse, and reversal of Sema3A-induced repulsion into attraction. We conclude that MARCKS is involved in regulating growth cone adhesion as follows: its nonphosphorylated form stabilizes integrin-mediated adhesions, and its phosphorylation-triggered release from adhesions causes localized growth cone detachment critical for turning and collapse.
Figures







References
-
- Aizawa H., et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat. Neurosci. 2001;4:367–373. - PubMed
-
- Barberis D., Artigiani S., Casazza A., Corso S., Giordano S., Love C. A., Jones E. Y., Comoglio P. M., Tamagnone L. Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. FASEB J. 2004;18:592–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

