Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV
- PMID: 16987970
- PMCID: PMC1642592
- DOI: 10.1128/JVI.01016-06
Human cytomegalovirus (HCMV) infection of endothelial cells promotes naive monocyte extravasation and transfer of productive virus to enhance hematogenous dissemination of HCMV
Abstract
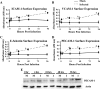
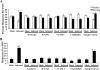
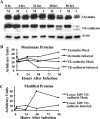
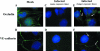
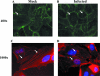
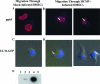
Human cytomegalovirus (HCMV) pathogenesis is dependent on the hematogenous spread of the virus to host tissue. While data suggest that infected monocytes are required for viral dissemination from the blood to the host organs, infected endothelial cells are also thought to contribute to this key step in viral pathogenesis. We show here that HCMV infection of endothelial cells increased the recruitment and transendothelial migration of monocytes. Infection of endothelial cells promoted the increased surface expression of cell adhesion molecules (intercellular cell adhesion molecule 1, vascular cell adhesion molecule 1, E-selectin, and platelet endothelial cell adhesion molecule 1), which were necessary for the recruitment of naïve monocytes to the apical surface of the endothelium and for the migration of these monocytes through the endothelial cell layer. As a mechanism to account for the increased monocyte migration, we showed that HCMV infection of endothelial cells increased the permeability of the endothelium. The cellular changes contributing to the increased permeability and increased naïve monocyte transendothelial migration include the disruption of actin stress fiber formation and the decreased expression of lateral junction proteins (occludin and vascular endothelial cadherin). Finally, we showed that the migrating monocytes were productively infected with the virus, documenting that the virus was transferred to the migrating monocyte during passage through the lateral junctions. Together, our results provide evidence for an active role of the infected endothelium in HCMV dissemination and pathogenesis.
Figures




References
-
- Adam, E., J. L. Melnick, J. L. Probtsfield, B. L. Petrie, J. Burek, K. R. Bailey, C. H. McCollum, and M. E. DeBakey. 1987. High levels of cytomegalovirus antibody in patients requiring vascular surgery for atherosclerosis. Lancet ii:291-293. - PubMed
-
- Berman, M. E., Y. Xie, and W. A. Muller. 1996. Roles of platelet/endothelial cell adhesion molecule-1 (PECAM-1, CD31) in natural killer cell transendothelial migration and beta 2 integrin activation. J. Immunol. 156:1515-1524. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

