Schwann cells express motor and sensory phenotypes that regulate axon regeneration
- PMID: 16988035
- PMCID: PMC6674436
- DOI: 10.1523/JNEUROSCI.1620-06.2006
Schwann cells express motor and sensory phenotypes that regulate axon regeneration
Abstract
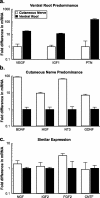
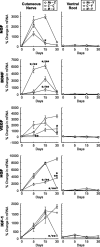
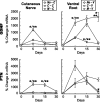
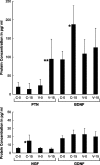
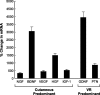
Schwann cell phenotype is classified as either myelinating or nonmyelinating. Additional phenotypic specialization is suggested, however, by the preferential reinnervation of muscle pathways by motoneurons. To explore potential differences in growth factor expression between sensory and motor nerve, grafts of cutaneous nerve or ventral root were denervated, reinnervated with cutaneous axons, or reinnervated with motor axons. Competitive reverse transcription-PCR was performed on normal cutaneous nerve and ventral root and on graft preparations 5, 15, and 30 d after surgery. mRNA for nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor, hepatocyte growth factor, and insulin-like growth factor-1 was expressed vigorously by denervated and reinnervated cutaneous nerve but minimally by ventral root. In contrast, mRNA for pleiotrophin (PTN) and glial cell line-derived neurotrophic factor was upregulated to a greater degree in ventral root. ELISA confirmed that NGF and BDNF protein were significantly more abundant in denervated cutaneous nerve than in denervated ventral root, but that PTN protein was more abundant in denervated ventral root. The motor phenotype was not immutable and could be modified toward the sensory phenotype by prolonged reinnervation of ventral root by cutaneous axons. Retrograde labeling to quantify regenerating neurons demonstrated that cutaneous nerve preferentially supported cutaneous axon regeneration, whereas ventral root preferentially supported motor axon regeneration. Schwann cells thus express distinct sensory and motor phenotypes that are associated with the support of regeneration in a phenotype-specific manner. These findings suggest that current techniques of bridging gaps in motor and mixed nerve with cutaneous graft could be improved by matching axon and Schwann cell properties.
Figures




References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- An J, Beauchemin N, Albanese J, Abney TO, Sullivan AK. Use of a rat cDNA probe specific for the Y chromosome to detect male-derived cells. J Androl. 1997;18:289–293. - PubMed
-
- Bernardo Corte MJ, Suarez Nieto C, Ablanedo Ablanedo P. Motor and sensory facial nerve grafts. An experimental comparative study. Arch Otolaryngol. 1984;110:378–383. - PubMed
-
- Blondet B, Carpentier G, Lafdil F, Courty J. Pleiotrophin cellular localization in nerve regeneration after peripheral nerve injury. J Histochem Cytochem. 2005;53:971–977. - PubMed
-
- Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27:277–324. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
