Identification of OmpU of Vibrio vulnificus as a fibronectin-binding protein and its role in bacterial pathogenesis
- PMID: 16988233
- PMCID: PMC1594942
- DOI: 10.1128/IAI.00171-06
Identification of OmpU of Vibrio vulnificus as a fibronectin-binding protein and its role in bacterial pathogenesis
Abstract
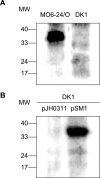
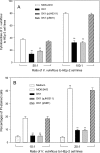
Vibrio vulnificus is a pathogenic bacterium that causes gastroenteritis and primary septicemia. To identify factors involved in microbial adherence to the host cells, we investigated bacterial proteins capable of binding to fibronectin, one of the main components comprised of the extracellular matrix of mammalian cells. A protein of approximately 35 kDa was purified from the extracts of V. vulnificus by its property to bind to immobilized fibronectin. This protein was identified as OmpU, one of the major outer membrane proteins of V. vulnificus. In binding assays using immobilized fibronectin, the number of ompU mutant cells bound to fibronectin was only 4% of that of wild-type cells bound to fibronectin. In addition, the exogenous addition of antibodies against OmpU resulted in a decreased ability of wild-type V. vulnificus to adhere to fibronectin. The ompU mutant was also defective in its adherence to RGD tripeptide (5% of the adherence of the wild type to RGD), cytoadherence to HEp-2 cells (7% of the adherence of the wild type to HEp-2), cytotoxicity to cell cultures (39% of the cytotoxicity of the wild type), and mortality in mice (10-fold increase in the 50% lethal dose). The ompU mutant complemented with the intact ompU gene restored its abilities for adherence to fibronectin, RGD tripeptide, and HEp-2 cells; cytotoxicity to HEp-2 cells; and mouse lethality. This study indicates that OmpU is an important virulence factor involved in the adherence of V. vulnificus to the host cells.
Figures






References
-
- Bahrani, K., and J. D. Oliver. 1990. Studies on the lipopolysaccharide of virulent and avirulent strains of Vibrio vulnificus. Biochem. Cell Biol. 68:547-551. - PubMed
-
- Connell, T. D., A. J. Martone, and R. K. Holmes. 1995. A new mobilizable cosmid vector for use in Vibrio cholerae and other gram-negative bacteria. Gene 153:85-87. - PubMed
-
- Crawford, J. A., J. B. Kaper, and V. J. DiRita. 1998. Analysis of ToxR-dependent transcription activation of ompU, the gene encoding a major envelope protein in Vibrio cholerae. Mol. Microbiol. 29:235-246. - PubMed
-
- Di Martino, P. 2001. Effects of antibiotics on adherence of Pseudomonas aeruginosa and Pseudomonas fluorescens to human fibronectin. Chemotherapy 47:344-349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

