Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval strongyloides stercoralis in mice
- PMID: 16988250
- PMCID: PMC1594891
- DOI: 10.1128/IAI.01958-05
Role of eosinophils and neutrophils in innate and adaptive protective immunity to larval strongyloides stercoralis in mice
Abstract
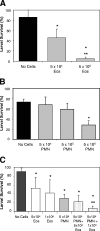
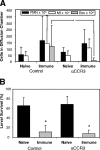
The goal of this study was to determine the roles of eosinophils and neutrophils in innate and adaptive protective immunity to larval Strongyloides stercoralis in mice. The experimental approach used was to treat mice with an anti-CCR3 monoclonal antibody to eliminate eosinophils or to use CXCR2-/- mice, which have a severe neutrophil recruitment defect, and then determine the effect of the reduction or elimination of the particular cell type on larval killing. It was determined that eosinophils killed the S. stercoralis larvae in naïve mice, whereas these cells were not required for the accelerated killing of larvae in immunized mice. Experiments using CXCR2-/- mice demonstrated that the reduction in recruitment of neutrophils resulted in significantly reduced innate and adaptive protective immunity. Protective antibody developed in the immunized CXCR2-/- mice, thereby demonstrating that neutrophils were not required for the induction of the adaptive protective immune response. Moreover, transfer of neutrophil-enriched cell populations recovered from either wild-type or CXCR2-/- mice into diffusion chambers containing larvae demonstrated that larval killing occurred with both cell populations when the diffusion chambers were implanted in immunized wild-type mice. Thus, the defect in the CXCR2-/- mice was a defect in the recruitment of the neutrophils and not a defect in the ability of these cells to kill larvae. This study therefore demonstrated that both eosinophils and neutrophils are required in the protective innate immune response, whereas only neutrophils are necessary for the protective adaptive immune response to larval S. stercoralis in mice.
Figures



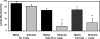
Similar articles
-
Strongyloides stercoralis: eosinophil-dependent immune-mediated killing of third stage larvae in BALB/cByJ mice.Exp Parasitol. 1996 Apr;82(3):267-78. doi: 10.1006/expr.1996.0034. Exp Parasitol. 1996. PMID: 8631378
-
Major basic protein from eosinophils and myeloperoxidase from neutrophils are required for protective immunity to Strongyloides stercoralis in mice.Infect Immun. 2011 Jul;79(7):2770-8. doi: 10.1128/IAI.00931-10. Epub 2011 Apr 11. Infect Immun. 2011. PMID: 21482685 Free PMC article.
-
Role of IL-5 in innate and adaptive immunity to larval Strongyloides stercoralis in mice.J Immunol. 2000 Oct 15;165(8):4544-51. doi: 10.4049/jimmunol.165.8.4544. J Immunol. 2000. PMID: 11035095
-
Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model.Immunol Res. 2011 Dec;51(2-3):205-14. doi: 10.1007/s12026-011-8258-2. Immunol Res. 2011. PMID: 22101674 Free PMC article. Review.
-
Strongyloides infection in rodents: immune response and immune regulation.Parasitology. 2017 Mar;144(3):295-315. doi: 10.1017/S0031182016000111. Epub 2016 Feb 24. Parasitology. 2017. PMID: 26905057 Review.
Cited by
-
Role of Macrophages in the Repair Process during the Tissue Migrating and Resident Helminth Infections.Biomed Res Int. 2016;2016:8634603. doi: 10.1155/2016/8634603. Epub 2016 Aug 25. Biomed Res Int. 2016. PMID: 27648452 Free PMC article. Review.
-
Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity.Mol Cell Biol. 2007 Aug;27(16):5699-710. doi: 10.1128/MCB.00383-07. Epub 2007 Jun 11. Mol Cell Biol. 2007. PMID: 17562860 Free PMC article.
-
ASCVac-1, a Multi-Peptide Chimeric Vaccine, Protects Mice Against Ascaris suum Infection.Front Immunol. 2021 Dec 21;12:788185. doi: 10.3389/fimmu.2021.788185. eCollection 2021. Front Immunol. 2021. PMID: 34992603 Free PMC article.
-
House dust mite sensitization drives cross-reactive immune responses to homologous helminth proteins.PLoS Pathog. 2021 Mar 2;17(3):e1009337. doi: 10.1371/journal.ppat.1009337. eCollection 2021 Mar. PLoS Pathog. 2021. PMID: 33651853 Free PMC article.
-
Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion.Nat Immunol. 2014 Oct;15(10):938-46. doi: 10.1038/ni.2984. Epub 2014 Aug 31. Nat Immunol. 2014. PMID: 25173346 Free PMC article.
References
-
- Abraham, D., H. L. Rotman, H. F. Haberstroh, W. Yutanawiboonchai, R. A. Brigandi, O. Leon, T. J. Nolan, and G. A. Schad. 1995. Strongyloides stercoralis: protective immunity to third-stage larvae in BALB/cByJ mice. Exp. Parasitol. 80:297-307. - PubMed
-
- Al-Qaoud, K. M., E. Pearlman, T. Hartung, J. Klukowski, B. Fleischer, and A. Hoerauf. 2000. A new mechanism for IL-5-dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int. Immunol. 12:899-908. - PubMed
-
- Balish, E., R. D. Wagner, A. Vazquez-Torres, J. Jones-Carson, C. Pierson, and T. Warner. 1999. Mucosal and systemic candidiasis in IL-8Rh−/− BALB/c mice. J. Leukoc. Biol. 66:144-150. - PubMed
-
- Banerjee, K., P. S. Biswas, B. Kim, S. Lee, and B. T. Rouse. 2004. CXCR2−/− mice show enhanced susceptibility to herpetic stromal keratitis: a role for IL-6-induced neovascularization. J. Immunol. 172:1237-1245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

