A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge
- PMID: 16988273
- PMCID: PMC1594937
- DOI: 10.1128/IAI.00590-06
A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against Plasmodium falciparum sporozoite challenge
Abstract
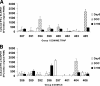
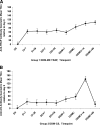
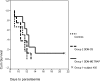
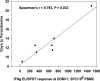
The safety, immunogenicity, and efficacy of DNA and modified vaccinia virus Ankara (MVA) prime-boost regimes were assessed by using either thrombospondin-related adhesion protein (TRAP) with a multiple-epitope string ME (ME-TRAP) or the circumsporozoite protein (CS) of Plasmodium falciparum. Sixteen healthy subjects who never had malaria (malaria-naive subjects) received two priming vaccinations with DNA, followed by one boosting immunization with MVA, with either ME-TRAP or CS as the antigen. Immunogenicity was assessed by ex vivo gamma interferon (IFN-gamma) enzyme-linked immunospot assay (ELISPOT) and antibody assay. Two weeks after the final vaccination, the subjects underwent P. falciparum sporozoite challenge, with six unvaccinated controls. The vaccines were well tolerated and immunogenic, with the DDM-ME TRAP regimen producing stronger ex vivo IFN-gamma ELISPOT responses than DDM-CS. One of eight subjects receiving the DDM-ME TRAP regimen was completely protected against malaria challenge, with this group as a whole showing significant delay to parasitemia compared to controls (P = 0.045). The peak ex vivo IFN-gamma ELISPOT response in this group correlated strongly with the number of days to parasitemia (P = 0.033). No protection was observed in the DDM-CS group. Prime-boost vaccination with DNA and MVA encoding ME-TRAP but not CS resulted in partial protection against P. falciparum sporozoite challenge in the present study.
Figures

References
-
- Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. - PubMed
-
- Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 366:2012-2018. - PubMed
-
- Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoitie, F. Dubovsky, C. Menendez, N. Tornieporth, W. R. Ballou, R. Thompson, and J. Cohen. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364:1411-1420. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Andrews, L., R. F. Andersen, D. Webster, S. Dunachie, R. M. Walther, P. Bejon, A. Hunt-Cooke, G. Bergson, F. Sanderson, A. V. Hill, and S. C. Gilbert. 2005. Quantitative real-time polymerase chain reaction for malaria diagnosis and its use in malaria vaccine clinical trials. Am. J. Trop. Med. Hyg. 73:191-198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

