Immunogenicity of Duffy binding-like domains that bind chondroitin sulfate A and protection against pregnancy-associated malaria
- PMID: 16988275
- PMCID: PMC1594931
- DOI: 10.1128/IAI.00481-06
Immunogenicity of Duffy binding-like domains that bind chondroitin sulfate A and protection against pregnancy-associated malaria
Abstract
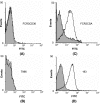
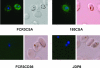
Sequestration of Plasmodium falciparum-infected erythrocytes in the placenta is implicated in pathological outcomes of pregnancy-associated malaria (PAM). P. falciparum isolates that sequester in the placenta primarily bind chondroitin sulfate A (CSA). Following exposure to malaria during pregnancy, women in areas of endemicity develop immunity, and so multigravid women are less susceptible to PAM than primigravidae. Protective immunity to PAM is associated with the development of antibodies that recognize diverse CSA-binding, placental P. falciparum isolates. The epitopes recognized by such protective antibodies have not been identified but are likely to lie in conserved Duffy binding-like (DBL) domains, encoded by var genes, that bind CSA. Immunization of mice with the CSA-binding DBL3gamma domain encoded by var1CSA elicits cross-reactive antibodies that recognize diverse CSA-binding P. falciparum isolates and block their binding to placental cryosections under flow. However, CSA-binding isolates primarily express var2CSA, which does not encode any DBLgamma domains. Here, we demonstrate that antibodies raised against DBL3gamma encoded by var1CSA cross-react with one of the CSA-binding domains, DBL3X, encoded by var2CSA. This explains the paradoxical observation made here and earlier that anti-rDBL3gamma sera recognize CSA-binding isolates and provides evidence for the presence of conserved, cross-reactive epitopes in diverse CSA-binding DBL domains. Such cross-reactive epitopes within CSA-binding DBL domains can form the basis for a vaccine that provides protection against PAM.
Figures
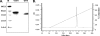
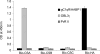
References
-
- Achur, R. N., M. Vialyavetti, A. Alkhalil, C. F. Ockenhouse, and D. C. Gowda. 2000. Characterization of proteoglycans of human placenta and identification of unique chondroitin sulfate proteoglycans of the intervillous spaces that mediate the adherence of Plasmodium falciparum-infected erythrocytes to the placenta. J. Biol. Chem. 275:40344-40356. - PubMed
-
- Avril, M., B. Traore, F. T. M. Costa, C. Lepolard, and J. Gysin. 2004. Placenta cryosection for study of the adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A in flow conditions. Microbes Infect. 6:249-255. - PubMed
-
- Baruch, D. I., B. L. Pasloske, H. B. Singh, X. Bi, X. C. Ma, M. Feldman, T. F. Taraschi, and R. J. Taraschi. 1997. Cloning the Plasmodium falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82:77-87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

