Taspine: bioactivity-guided isolation and molecular ligand-target insight of a potent acetylcholinesterase inhibitor from Magnolia x soulangiana
- PMID: 16989531
- PMCID: PMC3526713
- DOI: 10.1021/np060268p
Taspine: bioactivity-guided isolation and molecular ligand-target insight of a potent acetylcholinesterase inhibitor from Magnolia x soulangiana
Abstract
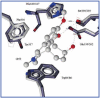
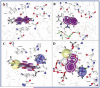
A bioactivity-guided approach was taken to identify the acetylcholinesterase (AChE, EC 3.1.1.7) inhibitory agent in a Magnolia x soulangiana extract using a microplate enzyme assay with Ellman's reagent. This permitted the isolation of the alkaloids taspine (1) and (-)-asimilobine (2), which were detected for the first time in this species. Compound 1 showed a significantly higher effect on AChE than the positive control galanthamine and selectively inhibited the enzyme in a long-lasting and concentration-dependent fashion with an IC(50) value of 0.33 +/- 0.07 muM. Extensive molecular docking studies were performed with human and Torpedo californica-AChE employing Gold software to rationalize the binding interaction. The results suggested ligand 1 to bind in an alternative binding orientation when compared to galanthamine. While this is located in close vicinity to the catalytic amino acid triad, the 1-AChE complex was found to be stabilized by (i) sandwich-like pi-stacking interactions between the planar aromatic ligand (1) and the Trp84 and Phe330 of the enzyme, (ii) an esteratic site anchoring with the amino side chain, and (iii) a hydrogen-bonding network.
Figures

Similar articles
-
The complex of a bivalent derivative of galanthamine with torpedo acetylcholinesterase displays drastic deformation of the active-site gorge: implications for structure-based drug design.J Am Chem Soc. 2004 Dec 1;126(47):15405-11. doi: 10.1021/ja0466154. J Am Chem Soc. 2004. PMID: 15563167
-
3D structure of Torpedo californica acetylcholinesterase complexed with huprine X at 2.1 A resolution: kinetic and molecular dynamic correlates.Biochemistry. 2002 Mar 5;41(9):2970-81. doi: 10.1021/bi011652i. Biochemistry. 2002. PMID: 11863435
-
Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts.Life Sci. 2002 Oct 11;71(21):2521-9. doi: 10.1016/s0024-3205(02)02034-9. Life Sci. 2002. PMID: 12270757
-
Molecular interaction of human brain acetylcholinesterase with a natural inhibitor huperzine-B: an enzoinformatics approach.CNS Neurol Disord Drug Targets. 2014 Apr;13(3):487-90. doi: 10.2174/18715273113126660163. CNS Neurol Disord Drug Targets. 2014. PMID: 24059299 Review.
-
Chemical and molecular aspects on interactions of galanthamine and its derivatives with cholinesterases.Curr Pharm Biotechnol. 2015;16(3):252-8. doi: 10.2174/1389201015666141202105105. Curr Pharm Biotechnol. 2015. PMID: 25483718 Review.
Cited by
-
In vitro opioid receptor affinity and in vivo behavioral studies of Nelumbo nucifera flower.J Ethnopharmacol. 2015 Nov 4;174:57-65. doi: 10.1016/j.jep.2015.08.006. Epub 2015 Aug 7. J Ethnopharmacol. 2015. PMID: 26260436 Free PMC article.
-
Phytochemical Screening of Rosmarinus officinalis L. as a Potential Anticholinesterase and Antioxidant-Medicinal Plant for Cognitive Decline Disorders.Plants (Basel). 2022 Feb 14;11(4):514. doi: 10.3390/plants11040514. Plants (Basel). 2022. PMID: 35214846 Free PMC article.
-
Small Molecule Compounds of Natural Origin Target Cellular Receptors to Inhibit Cancer Development and Progression.Int J Mol Sci. 2022 Feb 28;23(5):2672. doi: 10.3390/ijms23052672. Int J Mol Sci. 2022. PMID: 35269825 Free PMC article. Review.
-
In silico target fishing for rationalized ligand discovery exemplified on constituents of Ruta graveolens.Planta Med. 2009 Feb;75(3):195-204. doi: 10.1055/s-0028-1088397. Epub 2008 Dec 18. Planta Med. 2009. PMID: 19096995 Free PMC article.
-
Cyclodextrins Can Entrap Zearalenone-14-Glucoside: Interaction of the Masked Mycotoxin with Cyclodextrins and Cyclodextrin Bead Polymer.Biomolecules. 2019 Aug 9;9(8):354. doi: 10.3390/biom9080354. Biomolecules. 2019. PMID: 31405003 Free PMC article.
References
-
- Bartus RT, Dean RL, Beer B, Lippa AS. Science. 1982;217:408–414. - PubMed
-
- Zimmermann M, Gardoni F, Marcello E, Colciaghi F, Borroni B, Padovani A, Cattabeni F, Di Luca M. J. Neurochem. 2004;90:1489–1499. - PubMed
-
- Zimmermann M, Gardoni F, Di Luca M. Drugs Aging. 2005;22:27–37. - PubMed
-
- Jiang H, Luo X, Bai D. Curr. Med. Chem. 2003;10:2231–2252. - PubMed
-
- Houghton PJ, Ren Y, Howes M-J. Nat. Prod. Rep. 2006;23:181–199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Research Materials
Miscellaneous

