Global expression of NGF promotes sympathetic axonal growth in CNS white matter but does not alter its parallel orientation
- PMID: 16989811
- PMCID: PMC2638215
- DOI: 10.1016/j.expneurol.2006.07.026
Global expression of NGF promotes sympathetic axonal growth in CNS white matter but does not alter its parallel orientation
Abstract
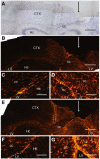
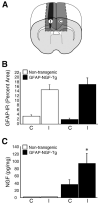
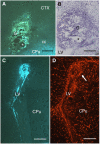
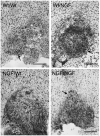
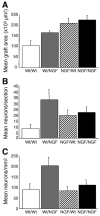
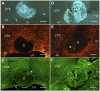
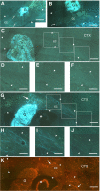
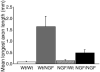
Axonal regeneration is normally limited after injuries to CNS white matter. Infusion of neurotrophins has been successful in promoting regenerative growth through injured white matter but this growth generally fails to extend beyond the infusion site. These observations are consistent with a chemotropic effect of these factors on axonal growth and support the prevailing view that neurotrophin-induced axonal regeneration requires the use of gradients, i.e., gradually increasing neurotrophin levels along the target fiber tract. To examine the potential of global overexpression of neurotrophins to promote, and/or modify the orientation of, regenerative axonal growth within white matter, we grafted nerve growth factor (NGF) responsive neurons into the corpus callosum of transgenic mice overexpressing NGF throughout the CNS under control of the promoter for glial fibrillary acidic protein. One week later, glial fibrillary acidic protein and chondroitin sulfate proteoglycan immunoreactivity increased within injured white matter around the grafts. NGF levels were significantly higher in the brains of transgenic compared with non-transgenic mice and further elevated within injury sites compared with the homotypic region of the non-injured side. Although there was minimal outgrowth from neurons grafted into non-transgenic mice, extensive parallel axonal regeneration had occurred within the corpus callosum up to 1.5 mm beyond the astrogliotic scar (the site of maximum NGF expression) in transgenic mice. These results demonstrate that global overexpression of neurotrophins does not override the constraints limiting regenerative growth to parallel orientations and suggest that such factors need not be presented as positive gradients to promote axonal regeneration within white matter.
Figures

Similar articles
-
A new in vitro model of the glial scar inhibits axon growth.Glia. 2008 Nov 15;56(15):1691-709. doi: 10.1002/glia.20721. Glia. 2008. PMID: 18618667 Free PMC article.
-
Relationship between sprouting axons, proteoglycans and glial cells following unilateral nigrostriatal axotomy in the adult rat.Neuroscience. 2002;109(1):101-17. doi: 10.1016/s0306-4522(01)00457-2. Neuroscience. 2002. PMID: 11784703
-
Nerve growth factor-hypersecreting Schwann cell grafts augment and guide spinal cord axonal growth and remyelinate central nervous system axons in a phenotypically appropriate manner that correlates with expression of L1.J Comp Neurol. 1999 Nov 1;413(4):495-506. doi: 10.1002/(sici)1096-9861(19991101)413:4<495::aid-cne1>3.0.co;2-z. J Comp Neurol. 1999. PMID: 10495438
-
Actions of neurotrophic factors and their signaling pathways in neuronal survival and axonal regeneration.Mol Neurobiol. 2006 Apr;33(2):155-79. doi: 10.1385/MN:33:2:155. Mol Neurobiol. 2006. PMID: 16603794 Review.
-
The bright side of the glial scar in CNS repair.Nat Rev Neurosci. 2009 Mar;10(3):235-41. doi: 10.1038/nrn2591. Nat Rev Neurosci. 2009. PMID: 19229242 Review.
Cited by
-
Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions.PLoS One. 2012;7(6):e39500. doi: 10.1371/journal.pone.0039500. Epub 2012 Jun 20. PLoS One. 2012. PMID: 22745769 Free PMC article.
-
Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation.Cell Commun Signal. 2009 Aug 27;7:20. doi: 10.1186/1478-811X-7-20. Cell Commun Signal. 2009. PMID: 19712465 Free PMC article.
-
The Role of Tissue Geometry in Spinal Cord Regeneration.Medicina (Kaunas). 2022 Apr 14;58(4):542. doi: 10.3390/medicina58040542. Medicina (Kaunas). 2022. PMID: 35454380 Free PMC article. Review.
-
Small molecule activators of the Trk receptors for neuroprotection.BMC Neurosci. 2008 Dec 3;9 Suppl 2(Suppl 2):S1. doi: 10.1186/1471-2202-9-S2-S1. BMC Neurosci. 2008. PMID: 19090982 Free PMC article. Review.
References
-
- Ayer-LeLievre C, Olson L, Ebendal T, seiger Å, Persson H. Expression of the β-nerve growth factor gene in hippocampal neurons. Science. 1988;240:1339–1341. - PubMed
-
- Bamber NI, Li H, Lu X, Oudega M, Aebischer P, Xu XM. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur. J. Neurosci. 2001;13:257–268. - PubMed
-
- Berry M, Rees L, hall S, Yiu P, Sievers J. Optic axons regenerate into sciatic nerve isografts only in the presence of Schwann cells. Brain Res. Bull. 1988;20:223–231. - PubMed
-
- Björklund A, Stenevi U. Growth of central catecholamine neurones into smooth muscle grafts in the rat mesencephalon. Brain Res. 1971;31:1–20. - PubMed
-
- Bradbury EJ, Khemani S, King VR, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur. J. Neurosci. 1999;11:3873–3883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

