Biological formation of ethane and propane in the deep marine subsurface
- PMID: 16990430
- PMCID: PMC1595412
- DOI: 10.1073/pnas.0606535103
Biological formation of ethane and propane in the deep marine subsurface
Abstract
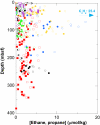
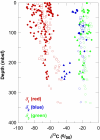
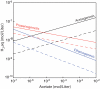
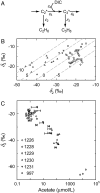
Concentrations and isotopic compositions of ethane and propane in cold, deeply buried sediments from the southeastern Pacific are best explained by microbial production of these gases in situ. Reduction of acetate to ethane provides one feasible mechanism. Propane is enriched in (13)C relative to ethane. The amount is consistent with derivation of the third C from inorganic carbon dissolved in sedimentary pore waters. At typical sedimentary conditions, the reactions yield free energy sufficient for growth. Relationships with competing processes are governed mainly by the abundance of H(2). Production of C(2) and C(3) hydrocarbons in this way provides a sink for acetate and hydrogen but upsets the general belief that hydrocarbons larger than methane derive only from thermal degradation of fossil organic material.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- D'Hondt SL, Jørgensen BB, Miller DJ, ODP Leg 201 Shipboard Scientific Party Proceedings of the Ocean Drilling Program; 2003. http://www-odp.tamu.edu/publications/201_IR/201ir.htm.
-
- D'Hondt SL, Jørgensen BB, Miller DJ, Batzke A, Blake R, Cragg BA, Cypionka H, Dickens GR, Ferdelman T, Hinrichs K-U, et al. Science. 2004;306:2216–2221. - PubMed
-
- Whiticar MJ, Suess E. Proc ODP Sci Res. 1990;112:527–538.
-
- Knies J, Damm E, Gutt J, Mann U, Pinturier L. Geochem Geophys Geosyst. 2004;5 doi: 10.1029/2003GC000687. - DOI
-
- Rowe D, Muehlenbachs A. Nature. 1999;398:61–63.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

