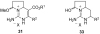Concise synthesis of guanidine-containing heterocycles using the Biginelli reaction
- PMID: 16995677
- PMCID: PMC2535792
- DOI: 10.1021/jo061199m
Concise synthesis of guanidine-containing heterocycles using the Biginelli reaction
Abstract
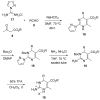
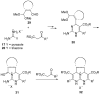
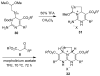
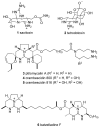
Two general methods for the synthesis of 2-imino-5-carboxy-3,4-dihydropyrimidines were developed using the three-component Biginelli reaction. The first method utilizes pyrazole carboxamidine, a beta-ketoester, and an aldehyde in an initial Biginelli reaction. After Boc protection, these products undergo aminolysis and acidic deprotection to generate 2-imino-5-carboxy-3,4-dihydropyrimidines in a four-step sequence. The second method utilizes a triazone-protected guanidine, a beta-ketoester, and an aldehyde in a Biginelli reaction. Acidic cleavage of the triazone yields 2-imino-5-carboxy-3,4-dihydropyrimidines in a two-step sequence. We also describe the further elaboration of several of these products using a tethered Biginelli reaction to give triazaacenaphthalene structures similar to those found in crambescidin and batzelladine alkaloids.
Figures
References
-
- Greenhill JV, Lue P. Prog. Med. Chem. 1993;30:203–326. - PubMed
-
-
For reviews of guanidine-containing natural products see:Berlinck RGS, Kossuga MH. Natl. Prod. Rep. 2005;22:516–550.Berlinck RGS. Natl. Prod. Rep. 2002;19:617–649.Faulkner DJ. Natl. Prod. Rep. 1999;16:155–198.Berlinck RGS. Natl. Prod. Rep. 1999;16:339–365.Berlinck RGS. Natl. Prod. Rep. 1996;13:377–409.
-
-
-
Arndt H-D, Koert U. Organic Synthesis Highlights IV. 2000:241–250.and references contained therein.
-
-
- Kashman Y, Hirsh S, McConnell OJ, Ohtani I, Kusumi T, Kakisawa H. J. Am. Chem. Soc. 1989;111:8925–8926.
- Ohtani I, Kusumi T, Kakisawa H, Kashman Y, Hirsh S. J. Am. Chem. Soc. 1992;114:8372–8479.
- Jares-Erijman EA, Sakai R, Rinehart KL. J. Org. Chem. 1991;56:5712–5715.
- Jares-Erijman EA, Ingrum AL, Carney JR, Rinehart KL, Sakai R. J. Org. Chem. 1993;58:4805–4808.
- Rinehart KL, Jares-Erijman EA. U.S. Patent 5,756,734. 1998.
- Shi J-G, Sun F, Rinehart KL. WO Patent 3,756,734. 1998.
- Berlinck RGS, Braekman JC, Daloze D, Bruno I, Riccio R, Ferri S, Spampinato S, Speroni E. J. Nat. Prod. 1993;56:1007–1015. - PubMed
- Palagiano E, De Marino S, Minale L, Riccio R, Zollo F, Iorizzi M, Carré JB, Debitus C, Lucarain L, Provost J. Tetrahedron. 1995;51:3675–3682.
- Braekman JC, Daloze D, Tavares R, Hajdu E, Van Soest RWM. J. Nat. Prod. 2000;63:193–196. - PubMed
- Chang LC, Whittaker NF, Bewley CA. J. Nat. Prod. 2003;66:1490–1494. - PubMed
- Aoki S, Kong D, Matsui K, Kobayashi M. Anticancer Res. 2004;24:2325–2330. - PubMed
-
- Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, De Brosse C, Mai S, Truneh A, Faulkner DJ, Carte B, Breen AL, Hertzberg RP, Johnson RK, Westley JW, Potts BCM. J. Org. Chem. 1995;60:1182–1188.
- Patil AD, Freyer AJ, Taylor PB, Carté B, Zuber G, Johnson RK, Faulkner DJ. J. Org. Chem. 1997;62:1814–1819.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources