Pharmacokinetics of subcutaneously administered etanercept in subjects with psoriasis
- PMID: 16995864
- PMCID: PMC1636685
- DOI: 10.1111/j.1365-2125.2006.02581.x
Pharmacokinetics of subcutaneously administered etanercept in subjects with psoriasis
Abstract
Aims: To present the results of the pharmacokinetic analysis of the concentration-time profiles of etanercept, a soluble receptor tumour necrosis factor (TNF) antagonist, in more than 1300 subjects with psoriasis.
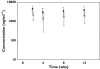
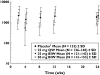
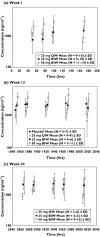
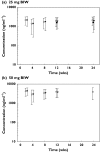
Methods: Pharmacokinetic samples were collected in one phase-2 and two phase-3 placebo-controlled, randomized clinical trials. Study 1 evaluated a 25-mg twice weekly (BIW) etanercept dosing regimen administered by subcutaneous (s.c.) injection for 24 weeks. Study 2 evaluated 25-mg BIW and 50-mg BIW s.c. doses for 12 weeks. Study 3 evaluated 25 mg once weekly (QW), 25 mg BIW and 50 mg BIW s.c. doses for 24 weeks.
Results: The mean +/- SD steady-state predose serum concentrations of etanercept for the 25-mg BIW arm at 12 weeks in study 1 were 1590 +/- 885 ng ml(-1). In study 2, mean +/- SD etanercept steady-state concentrations at 12 weeks were 1900 +/- 1110 ng ml(-1) in the 25-mg BIW group and 3830 +/- 1870 ng ml(-1) in the 50-mg BIW group. The mean +/- SD steady-state predose serum concentrations of etanercept at 12 weeks in study 3 were 768 +/- 475 ng ml(-1) for the 25-mg QW regimen, 1990 +/- 1030 ng ml(-1) for the 25-mg BIW regimen and 4020 +/- 2100 ng ml(-1) for the 50-mg BIW regimen.
Conclusions: Pharmacokinetic results were highly consistent across clinical trials. The concentration-time profiles displayed dose proportionality. Etanercept concentrations in subjects with psoriasis are similar to the concentrations in subjects with rheumatoid arthritis.
Figures
References
-
- Koo J. Population-based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin. 1996;14:485–96. - PubMed
-
- Granstein RD. New treatments for psoriasis. N Engl J Med. 2001;345:284–7. - PubMed
-
- Bonifati C, Ameglio F. Cytokines in psoriasis. Int J Dermatol. 1999;38:241–51. - PubMed
-
- Enbrel® (etanercept) Thousand Oaks, CA: Amgen Inc. & Wyeth-Ayerst Pharmaceuticals; 2004. [package insert].
-
- Moreland LW, Schiff MH, Baumgartner SW, Tindall EA, Fleischmann RM, Bulpitt KJ, Weaver AL, Keystone EC, Furst DE, Mease PJ, Ruderman EM, Horwitz DA, Arkfeld DG, Garrison L, Burge DJ, Blosch CM, Lange ML, McDonnell ND, Weinblatt ME. Etanercept therapy in rheumatoid arthritis. A randomized, controlled trial. Ann Intern Med. 1999;130:478–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

